Question: Lead nitrate and potassium iodide react in aqueous solution to form a yellow precipitate of lead iodide. In one series of experiments, the masses of
Lead nitrate and potassium iodide react in aqueous solution to form a yellow precipitate of lead iodide. In one series of experiments, the masses of the two reactants were varied, but the total mass of the two was held constant at 5.000 g. The lead iodide formed was filtered from solution, washed, dried, and weighed. The table gives data for a series of reactions.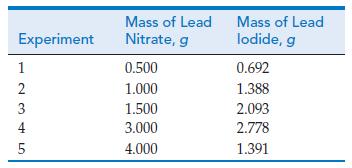
(a) Plot the data in a graph of mass of lead iodide versus mass of lead nitrate, and draw the appropriate curve(s) connecting the data points. What is the maximum mass of precipitate that can be obtained?
(b) Explain why the maximum mass of precipitate is obtained when the reactants are in their stoichiometric proportions. What are these stoichiometric proportions expressed as a mass ratio, and as a mole ratio?
(c) Show how the stoichiometric proportions determined in part (b) are related to the balanced equation for the reaction.
Experiment 12345 Mass of Lead Nitrate, g 0.500 1.000 1.500 3.000 4.000 Mass of Lead lodide, g 0.692 1.388 2.093 2.778 1.391
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


