One of the key reactions in the gasification of coal is the methanation reaction, in which methane
Question:
One of the key reactions in the gasification of coal is the methanation reaction, in which methane is produced from synthesis gas—a mixture of CO and H2.
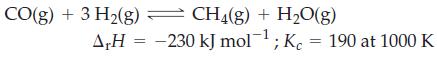
(a) Is the equilibrium conversion of synthesis gas to methane favored at higher or lower temperatures? Higher or lower pressures?
(b) Assume you have 4.00 mol of synthesis gas with a 3 : 1 mole ratio of H2(g) to CO(g) in a 15.0 L flask. What will be the mole fraction of CH4(g) at equilibrium at 1000 K?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





