Radioactive decay and mass spectrometry are often used to date rocks after they have cooled from a
Question:
Radioactive decay and mass spectrometry are often used to date rocks after they have cooled from a magma. 87Rb has a half-life of 4.88 x 1010 years and follows the radioactive decay
![]()
A rock was dated by assaying the product of this decay. The mass spectrum of a homogenized sample of rock showed the 87Sr/86Sr ratio to be 2.25. Assume that the original 87Sr/86Sr ratio was 0.700 when the rock cooled. Chemical analysis of the rock gave 15.5 ppm Sr and 265.4 ppm Rb, using the average atomic masses from a periodic table. The other isotope ratios were 86Sr/88Sr = 0.119 and 84Sr/88Sr = 0.007. The isotopic ratio for 87Rb/85Rb is 0.330. The isotopic masses are as follows:
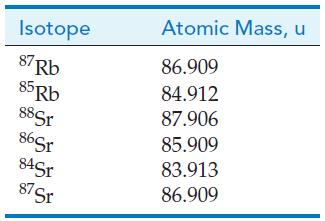
Calculate the following:
(a) The average atomic mass of Sr in the rock
(b) The original concentration of Rb in the rock in ppm
(c) The percentage of rubidium-87 decayed in the rock
(d) The time since the rock cooled.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





