Refer only to the periodic table on the inside front cover, and arrange the following ionization energies
Question:
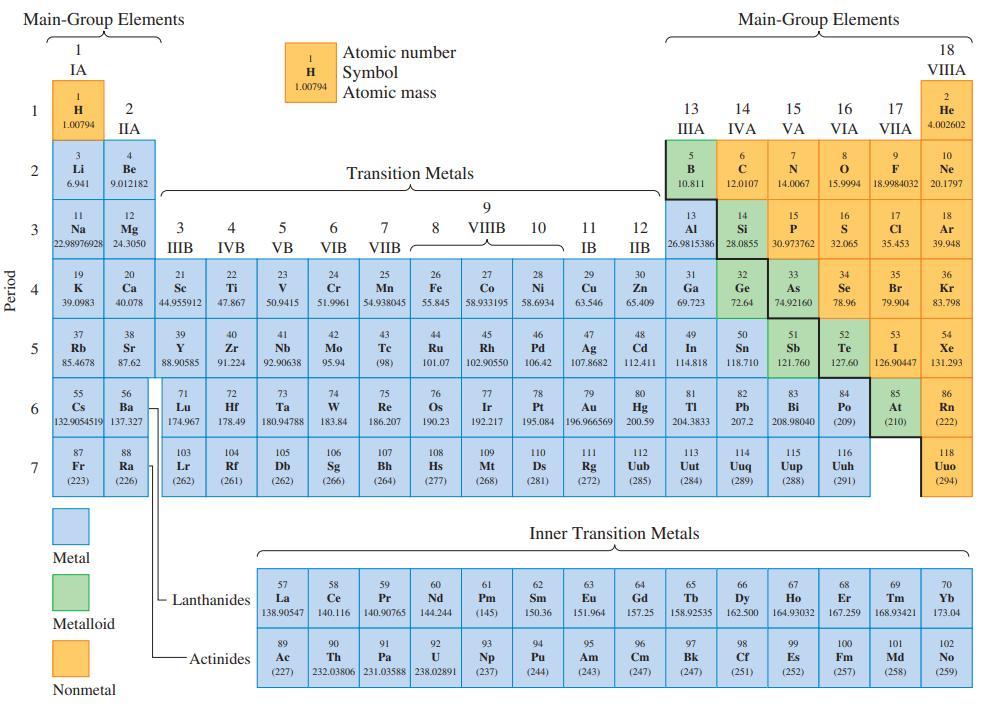
Refer only to the periodic table on the inside front cover, and arrange the following ionization energies in order of increasing value: the first ionization energy of F; the second ionization energy of Ba; the third ionization energy of Sc; the second ionization energy of Na; the third ionization energy of Mg. Explain the basis of any uncertainties.
Transcribed Image Text:
Period Main-Group Elements 1 2 4 5 11 3 Na 6 IA 7 1 H 1.00794 3 Li 6.941 19 K 39.0983 12 Mg 22.98976928 24.3050 37 Rb 85.4678 55 Cs 87 Fr (223) 4 Be 9.012182 Metal 2 IIA 56 Ba 132.9054519 137.327 Metalloid 20 Ca 40.078 38 Sr 87.62 Nonmetal 88 Ra (226) 3 IIIB 21 Se 44.955912 39 Y 88.90585 71 Lu 174.967 103 Lr (262) 4 IVB 22 Ti 47.867 40 Zr 91.224 72 Hf 178.49 104 Rf (261) Lanthanides Actinides 5 VB 23 V 50.9415 1 H Symbol 1.00794 Atomic mass 41 Nb 92.90638 73 Ta 180,94788 105 Db (262) Atomic number 6 VIB Transition Metals 24 Cr 51.9961 42 Mo 95.94 74 W 183.84 106 Sg (266) 57 58 La Ce 138.90547 140.116 7 8 VIIB 25 Mn 54.938045 43 Te (98) 75 Re 186.207 107 Bh (264) 59 Pr 140.90765 26 Fe 55.845 91 Pa 44 Ru 101.07 76 Os 190.23 108 Hs (277) 60 Nd 144.244 92 89 Ac (227) 232.03806 231.03588 238.02891 90 Th U 9 VIIIB 27 Co 58.933195 45 Rh 102.90550 77 Ir 192.217 109 Mt (268) 61 Pm (145) 93 Np (237) 10 28 Ni 58.6934 46 Pd 106.42 110 Ds (281) 11 12 IB IIB 78 79 Pt Au 195.084 196.966569 62 Sm 150.36 29 Cu 63.546 94 Pu (244) 47 Ag 107.8682 30 Zn 65.409 63 Eu 151.964 48 Cd 112.411 111 112 Rg Uub (272) (285) 95 Am (243) 80 Hg 200.59 64 Gd 157.25 13 IIIA 96 Cm (247) 5 B 10.811 Inner Transition Metals 31 Ga 69.723 49 In 114.818 13 14 Al Si 26.9815386 28.0855 81 TI 204.3833 113 Uut (284) 65 Tb 158.92535 Main-Group Elements 97 Bk (247) 14 IVA 6 с 12.0107 32 Ge 72.64 50 Sn 118.710 82 Pb 207.2 114 Uuq (289) 66 Dy 162.500 98 Cf (251) 15 VA 7 N 14.0067 15 P 30.973762 33 As 74.92160 51 Sb 121.760 83 Bi 208.98040 115 Uup (288) 67 Ho 164.93032 99 Es (252) 16 VIA 8 9 0 F 15.9994 18.9984032 16 S 32.065 34 Se 78.96 52 Te 127.60 84 Po (209) 116 Uuh (291) 68 Er 167.259 17 VIIA 100 Fm (257) 17 CI 35.453 35 Br 79.904 53 I 126.90447 85 At (210) 69 Tm 168.93421 101 Md (258) 18 VIIIA 2 He 4.002602 10 Ne 20.1797 18 Ar 39.948 36 Kr 83.798 54 Xe 131.293 86 Rn (222) 118 Uuo (294) 70 Yb 173.04 102 No (259)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The basis for the uncertainties in arranging the ionization energies in order of increasing value is that we are only looking at the periodic table and not using any other information The periodic tab...View the full answer

Answered By

HILLARY KIYAYI
I am a multi-skilled, reliable & talented Market analysis & Research Writer with a proven ability to produce Scholarly Papers, Reports, Research and Article Writing and much more. My ultimate quality is my English writing/verbal skill. That skill has proven to be the most valuable asset for project writing, Academic & Research writing, Proofreading, HR Management Writing, business, sales, and a variety of other opportunities.
4.80+
24+ Reviews
60+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer only to the periodic table on the inside front cover and indicate which of the atoms, Bi, S, Ba, As, and Ca, (a) Is most metallic; (b) Is most nonmetallic; (c) Has the intermediate value when...
-
(A) Refer only to the periodic table on the inside front cover, and arrange the following species in order of increasing size: Ti 2+ , V 3+ , Ca 2+ , Br - , and Sr 2+ . (B) Refer only to the periodic...
-
(A) Refer to the periodic table on the inside front cover, and arrange the following in the expected order of increasing first ionization energy Cl, K, Mg, S. (B) Refer to the periodic table on the...
-
As an agricultural engineer, you must design a trapezoidal open channel to carry irrigation water (Figure). Determine the optimal dimensions to minimize the wetted perimeter for a cross-sectional...
-
A partially completed balance sheet for Blue Co., Inc., as of January 31, 2011, follows. Where amounts are shown for various items, the amounts are correct. Required: Using the following data,...
-
Responsibility for the fixed cost volume variance Ragan Company expected to sell 400,000 of its pagers during 2011. It set the standard sales price for the pager at $30 each. During June, it became...
-
Your instructor will divide your class randomly into groups of four to six people. Acting as a team, with everyone offering ideas and one person serving as official recorder, each group will be...
-
(Computation of Basic and Diluted EPS) The information below pertains to Barkley Company for 2010. Net income for the year.................................................................$1,200,000...
-
Dan is standing a distance d = 25 m away from the base of a cliff of height H = 8 m. He is going to throw a baseball from 2 m above the ground at an angle = 53 above the horizontal with initial speed...
-
Use values of basic physical constants and other data from the appendices to show that 1 eV/atom = 96.49 kJ mol -1 .
-
An ion that is isoelectronic with Se 2- is (a) S 2- ; (b) I - ; (c) Xe; (d) Sr 2+ .
-
Find i and v for t = 0 and t = 0 + in the circuit of Fig. 7-16, given R = 5 , L = 10 mH, and V = [5V [5 sin cot (V) for t < 0 for t > 0
-
Repeat 4.21.1 but now use NOPs only when a hazard cannot be avoided by changing or rearranging these instructions. You can assume register R7 can be used to hold temporary values in your modified...
-
What are the various ways of setting promotional budgets? Comment on the strengths and weaknesses of each.
-
Describe the differences and similarities between spectators and participants of sport.
-
Ask 10 consumers about the value they believe a professional sports team would (or does) bring to the community. Then ask the same people about the value of college athletics to the community....
-
What are ISO 9001 and Six Sigma?
-
Depreciation calculation methods. Millco, Inc., acquired a machine that cost $400,000 early in 2013. The machine is expected to last for eight years, and its estimated salvage value at the end of its...
-
Assume you are the accountant for Catalina Industries. John Catalina, the owner of the company, is in a hurry to receive the financial statements for the year ended December 31, 20X1, and asks you...
-
A number of costs are listed below that may be relevant in decisions faced by the management of Svahn, AB, a Swedish manufacturer of sailing yachts: Required: Copy the information above onto your...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Management is concerned about the...
-
Troy Engines, Ltd., manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Julian and Casper were discussing how the CEO of their company recently managed a critical issue, which could have negatively impacted the company. They both stated that the CEO would normally ask...
-
The following schedule shows the Current Assets section of Water Source Company for the years ended December 31, 20Y8, and December 31, 20Y9: Water Source Company Comparative Schedule of Current...
-
Jones files a personal injury suit in state trial court and loses. He immediately demands his lawyer appeal to the U.S. Supreme Court. His lawyer explains he cannot do that. Why?

Study smarter with the SolutionInn App


