Shown below is a diagram of Boyles original apparatus. At the start of the experiment, the length
Question:
Shown below is a diagram of Boyle’s original apparatus. At the start of the experiment, the length of the
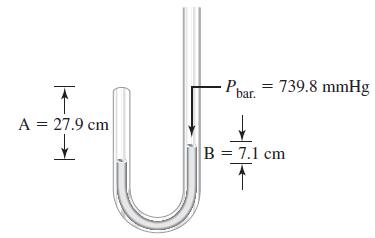
air column (A) on the left was 30.5 cm and the heights of mercury in the arms of the tube were equal. When mercury was added to the right arm of the tube, a difference in mercury levels (B) was produced, and the entrapped air on the left was compressed into a shorter length of the tube (smaller volume) as shown in the illustration for A = 27.9 cm and B = 7.1 cm. Boyle’s values of A and B, in centimeters, are listed as follows:
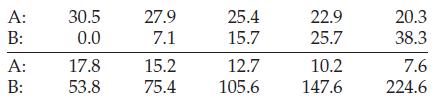
Barometric pressure at the time of the experiment was 739.8 mmHg. Assuming that the length of the air column (A) is proportional to the volume of air, show that these data conform reasonably well to Boyle’s law.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





