A particular equation of state for O 2 (g) has the form where V is the molar
Question:
A particular equation of state for O2(g) has the form
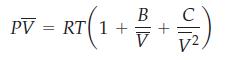
where V̅ is the molar volume, B = -21.89 cm3/mol and C = 1230 cm6/mol2.
(a) Use the equation to calculate the pressure exerted by 1 mol O2(g) confined to a volume of at 273 K.
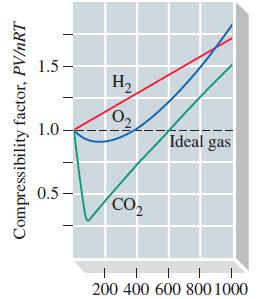
(b) Is the result calculated in part (a) consistent with that suggested for O2(g) by Figure 6-21? Explain.
Figure 6-21
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





