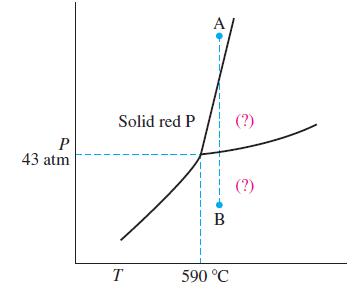
Shown here is a portion of the phase diagram for phosphorus. (a) Indicate the phases present in
Question:
Shown here is a portion of the phase diagram for phosphorus.
(a) Indicate the phases present in the regions labeled with a question mark.
(b) A sample of solid red phosphorus cannot be melted by heating in a container open to the atmosphere. Explain why this is so.
(c) Trace the phase changes that occur when the pressure on a sample is reduced from point A to B, at constant temperature..
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





