Sketch a titration curve (pH versus mL of titrant) for each of the following three hypothetical weak
Question:
Sketch a titration curve (pH versus mL of titrant) for each of the following three hypothetical weak acids when titrated with 0.100 M NaOH. Select suitable indicators for the titrations from Figure 17-7.
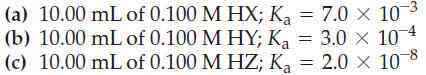
Figure 17-7
Transcribed Image Text:
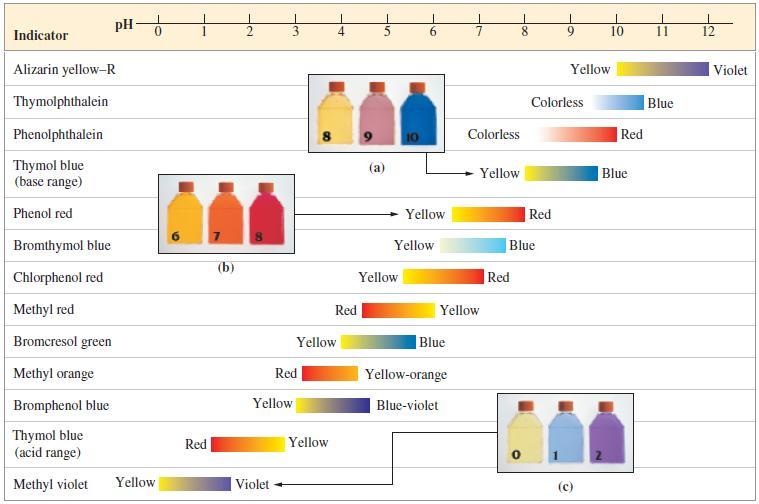
(a) 10.00 mL of 0.100 M HX; Ka = 7.0 x 10 (b) 10.00 mL of 0.100 M HY; Ka = 3.0 x 10-4 (c) 10.00 mL of 0.100 M HZ; K₂ = 2.0 × 10 Ka
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Sketch a titration curve (pH versus mL of titrant) for each of the following hypothetical weak bases when titrated with 0.100 M HCl. (Think of these bases as involving the substitution of organic...
-
At times, a salt of a weak base can be titrated by a strong base. Use appropriate data from the text to sketch a titration curve for the titration of 10.00 mL of 0.0500 M C 6 H 5 NH 3 + Cl - with...
-
Hrishi is a senior executive for a large manufacturing company in Mississauga Ontario where he has been employed for the past 10 years and his annual salary is $250,000 including bonus. He is 47...
-
Tantor Supply, Inc., is a small corporation acting as the exclusive distributor of a major line of sporting goods. During 2010 the firm earned $92,500 before taxes. a. Calculate the firm?s tax...
-
Condensed financial data of Sinjh Inc. follow. Additional information: 1. New plant assets costing $80,000 were purchased for cash during the year. 2. Old plant assets having an original cost of...
-
What do you like best about working at this company?
-
Candra Christensen Cuisine operates a chain of fine seafood restaurants. The company makes very detailed long-term planning. On October 1, 2011, Candra Christensen determined that it would need to...
-
Let U={1,2,3,...,10}, A={1,3,5,7}, B={1,2,3,4}, and C={3,4,6,7,9}. Find BC'
-
Determine the following characteristics of the titration curve for 20.0 mL of 0.275 M NH 3 (aq) titrated with 0.325 M HI(aq). (a) The initial pH; (b) The volume of 0.325 M HI(aq) at the equivalence...
-
Explain whether the equivalence point of each of the following titrations should be below, above, or at pH 7: (a) NaHCO 3 (aq) titrated with NaOH(aq); (b) HCl(aq) titrated with NH 3 (aq); (c) KOH(aq)...
-
What advantage do blogs have over the MSM? What advantage does the MSM have over the most popular blogs?
-
Find the present value of an annuity due that pays $3000 at the beginning of each quarter for the next 5 years. Assume that money is worth 6.6%, compounded quarterly. (Round your answer to the...
-
Leonard Co is expected to pay a dividend of $2.75. If this dividend is expected to grow at a 3% rate indefinitely and you require a return of 12%, A. what would you expect to pay for a share of...
-
A fabric factory has 5 weaving machines in use. These weaving machines need repair after about 2 0 hours of use. Breakdowns have been determined to be Poisson distributed. Jim, the maintenance worker...
-
How do perceived freedom and intrinsic motivation vary to explain leisure and non-leisure in Neulinger's Paradigm?
-
How do global health crises such as pandemics highlight the interconnectedness of nations and the need for coordinated responses?
-
Given her evaluation of current economic conditions, Ima Nutt believes there is a 20 percent probability of recession, a 50 percent chance of continued steady growth, and a 30 percent probability of...
-
Which provision could best be justified as encouraging small business? a. Ordinary loss allowed on $ 1244 stuck. b. Percentage depletion. c. Domestic production activates deductions. d. Interest...
-
From the following list, identify the accounts that should be closed to Income Summary at the end of the fiscal year: a. Accounts Receivable b. Accumulated DepreciationEquipment c. Depreciation...
-
Prior to its closing, Income Summary had total debits of $432,200 and total credits of $572,600. Briefly explain the purpose served by the income summary account and the nature of the entries that...
-
After all revenue and expense accounts have been closed at the end of the fiscal year, Income Summary has a debit of $193,400 and a credit of $258,600. At the same date, Laurie Engan, Capital has a...
-
8. Determine whether the set of vectors W = 5 8/ form a basis for R (7
-
This assignment assumes that you completed the hybrid activity in Lesson 1. If you have not done that, you will need to complete it before doing this assignment. Open a new Word document and place...
-
ERD Exercise - Database Management Systems Acme Autos has hired you to show a database to manage car sales at its five branches. Before building the database you conduct a preliminary analysis to...

Study smarter with the SolutionInn App


