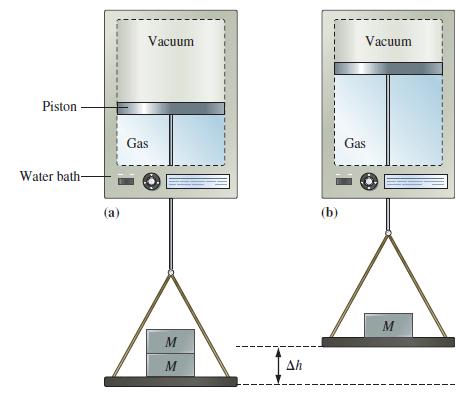
Suppose the gas in Figure 7-8 is 0.100 mol He at 298 K, the two weights correspond
Question:
Suppose the gas in Figure 7-8 is 0.100 mol He at 298 K, the two weights correspond to an external pressure of 2.40 atm in Figure 7-8(a), and the single weight in Figure 7-8(b) corresponds to an external pressure of 1.20 atm. How much work, in joules, is associated with the gas expansion at constant temperature?
Figure 7-8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





