The combustion of hydrogenoxygen mixtures is used to produce very high temperatures (approximately 2500 C) needed for
Question:
The combustion of hydrogen–oxygen mixtures is used to produce very high temperatures (approximately 2500 °C) needed for certain types of welding operations. Consider the reaction to be
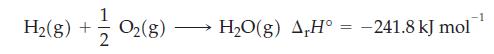
What is the quantity of heat evolved, in kilojoules, when a 180 g mixture containing equal parts of H2 and O2 by mass is burned?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





