The crystal structure of magnesium oxide, MgO, is of the NaCl type (Fig. 12-48). Use this fact,
Question:
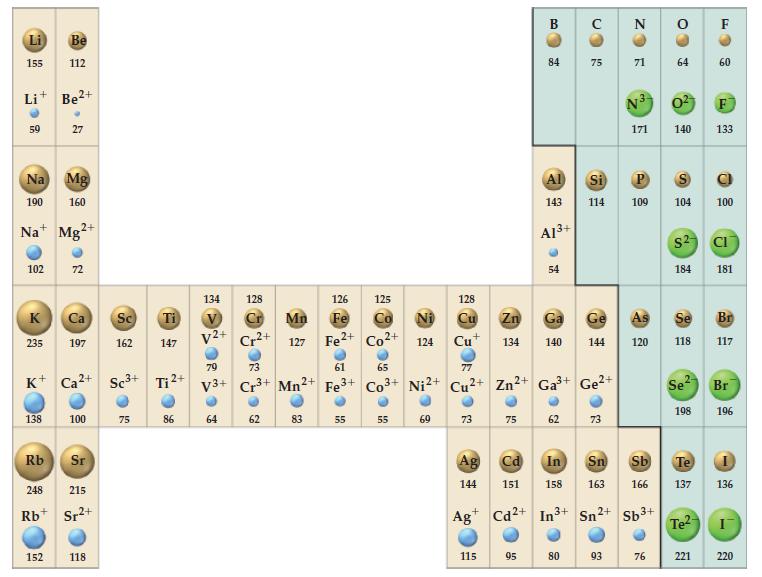
The crystal structure of magnesium oxide, MgO, is of the NaCl type (Fig. 12-48). Use this fact, together with ionic radii from Figure 9-11, to establish the following.
(a) The coordination numbers of Mg2+ and O2-;
(b) The number of formula units in the unit cell;
(c) The length and volume of a unit cell;
(d) The density of MgO.
Fig. 12-48
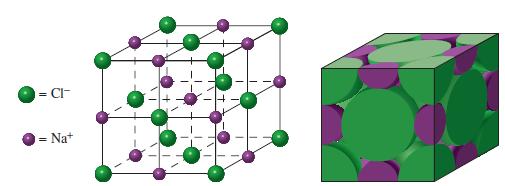
Figure 9-11
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





