The following r G values are given for 25 C. Combine the preceding equations, as necessary,
Question:
The following ΔrG° values are given for 25 °C.
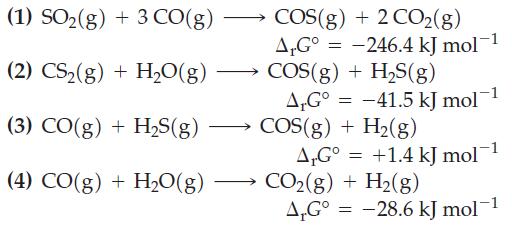
Combine the preceding equations, as necessary, to obtain ΔrG° values for the following reactions.
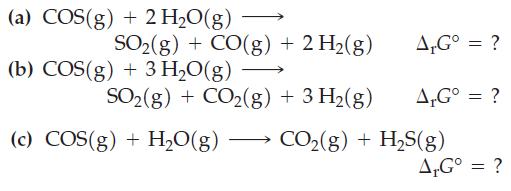
Of reactions (a), (b), and (c), which is spontaneous in the forward direction when reactants and products are present in their standard states?
Transcribed Image Text:
(1) SO₂(g) + 3 CO(g) (2) CS₂(g) +H₂O(g) (3) CO(g) + H₂S(g) (4) CO(g) + H₂O(g) COS(g) + 2 CO2(g) AG° = -246.4 kJ mol-1 COS(g) + H₂S(g) A,G° -41.5 kJ mol COS(g) + H₂(g) AG° +1.4 kJ mol-1 CO₂(g) + H₂(g) A.Gº = -28.6 kJ mol-¹ = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To obtain the ArG values for the given reactions you can sum the ArG values of the relevant reaction...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following r G values are given for 25 C. Combine the preceding equations, as necessary, to obtain r G values for each of the following reactions. Of reactions (a), (b), and (c), which would...
-
As the United States fell further and further into a recession in the years 2007-2009, the number of families who had to default on their mortgages and those who actually lost their homes grew....
-
A survey was conducted prior to the 2004 presidential election to explore the relationship between a persons religious fervor and their choice of a political candidate. Voters were asked how often...
-
Find an equation of the tangent plane at the given point. f(x, y) = x + y, (4,1)
-
What costs are excluded from the cost base when absorption-cost pricing is used to determine the markup percentage?
-
(a) By direct differentiation of H = U + P V, obtain a relation between (H/U)p and (U/V)p (b) Confirm that (H/U)p = I + p(V/U)p by expressing (H/U)p as the ratio of two derivatives with respect to...
-
Martin Kline, engagement partner for RCT Manufacturing Company, a February 28, 1999, year end client, is performing analytical procedures to better understand RCT's business and to determine where...
-
The manager of the Fore and Aft Marina in Problem 16 wants to investigate the possibility of enlarging the docking facility so that two boats can stop for gas and servicing simultaneously. Assume...
-
At December 31, 2024, Blossom Imports reported this information on its balance sheet. Accounts receivable $500,000 Less: Allowance for doubtful accounts 36,000 During 2025, the company had the...
-
Together with the following data, to estimate the bond-dissociation energy of the F 2 molecule. Compare your result with the value listed in Table 10.3. Table 10.3 F(g) - 2 F(g) A.G = 123.9 kJ mol-1
-
At 298 K, for the reaction 2 PCl 3 (g) + O 2 (g) 2 POCl 3 (l), r H = -620.2 kJ mol -1 and the standard molar entropies, in J mol 1 K 1 , are PCl 3 (g), 311.8; O 2 (g), 205.1; and POCl 3 (l), 222.4....
-
How do source reduction, reuse, and recycling reduce the volume of solid waste?
-
Christopher's Custom Cabinet Company uses a job order cost system with overhead applied as a percentage of direct labor costs. Inventory balances at the beginning of the current year follow: Raw...
-
What is one of the main differences between (1) a freehold estate and (2) a non-freehold estate?
-
Modern Cabinets has decided to price its cabinets at 70% above cost. The problem is that they aren't sure how to assign factory overhead. Your manager has asked you to price the following two jobs...
-
The Morrit Corporation has $1,020,000 of debt outstanding, and it pays an interest rate of 11% annually. Morrit's annual sales are $6 million, its average tax rate is 25%, and its net profit margin...
-
Strong Corporation had consistently used the cash method of accounting even though inventories were a material income-producing factor to its business and average annual gross receipts in the prior...
-
Lindas Foods produces frozen meals that it sells for $ 6 each. The company computes a new monthly fixed manufacturing overhead rate based on the planned number of meals to be produced that month. All...
-
Write the statement to store the contents of the txtAge control in an Integer variable named intAge.
-
High-low method Ken Howard, financial analyst at JVR Corporation, is examining the behavior of quarterly maintenance costs for budgeting purposes. Howard collects the following data on machine-hours...
-
High-low method and regression analysis. Happy Business College has recently opened a restaurant as part of its hospitality major. For the first 10 weeks the manager did not estimate any costs, but...
-
High-low method, regression analysis. Anna Martinez, the financial manager at the Casa Real restaurant is checking to see if there is any relationship between newspaper advertising and sales revenues...
-
1. A spring with a force constant of 600 N/m is used in a scale for weighing fish. What is the mass of a fish that stretches the spring 7.5 cm from its normal length? [2 marks] 2. What is the average...
-
How can cultural sensitivity be demonstrated in business reports and proposals, especially when dealing with international audiences or topics? Provide guidelines for respectful and inclusive...
-
How should follow-up actions and next steps be communicated in a business proposal? Explain the importance of outlining clear action items and the process for ensuring they are carried out.

Study smarter with the SolutionInn App


