The following r G values are given for 25 C. Combine the preceding equations, as necessary,
Question:
The following ΔrG° values are given for 25 °C.
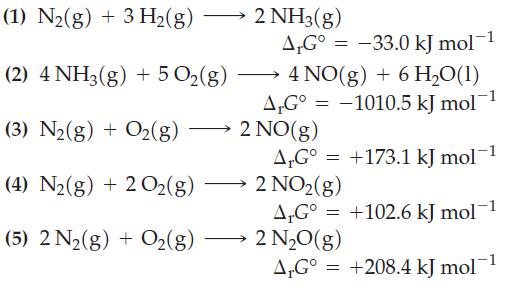
Combine the preceding equations, as necessary, to obtain ΔrG° values for each of the following reactions.
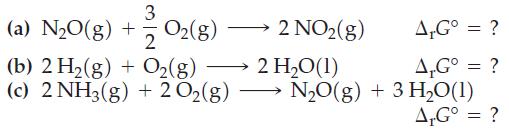
Of reactions (a), (b), and (c), which would tend to go to completion at 25 °C, and which would reach an equilibrium condition with significant amounts of all reactants and products present?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





