The following two equilibrium reactions can be written for aqueous carbonic acid, H 2 CO 3 (aq):
Question:
The following two equilibrium reactions can be written for aqueous carbonic acid, H2CO3(aq):
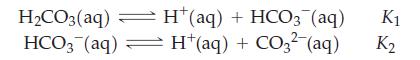
For each reaction write the equilibrium constant expression. By using Le Châtelier’s principle we may naively predict that by adding H2CO3 to the system, the concentration of CO32- would increase. What we observe is that after adding H2CO3 to the equilibrium mixture, an increase in the concentration of CO32- occurs when [CO32-] ≪ K2; however, the concentration of CO32- will decrease when [CO32-] W K2. Show that this is true by considering the ratio of [H+]/[HCO3-] before and after adding a small amount of H2CO3 to the solution, and by using that ratio to calculate the [CO32-].
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





