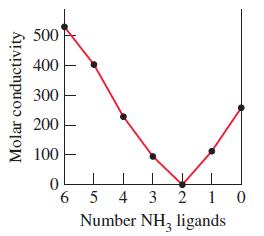
The graph that follows represents the molar conductivity of some Pt(IV) complexes. The ligands in these complexes
Question:
The graph that follows represents the molar conductivity of some Pt(IV) complexes. The ligands in these complexes are NH3 molecules or Cl– ions, the coordination number of Pt(IV) is 6, and the counter ions (for balancing charge) are K+ or Cl–.Write formulas for the coordination compounds corresponding to each point in the graph. (Molar conductivity is the electrical conductivity, under precisely defined conditions, of an aqueous solution containing one mole of a compound.)
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:




