The pH of ocean water depends on the amount of atmospheric carbon dioxide. The dissolution of carbon
Question:
The pH of ocean water depends on the amount of atmospheric carbon dioxide. The dissolution of carbon dioxide in ocean water can be approximated by the following chemical reactions (Henry’s Law constant for CO2 is KH = [CO2(aq)]/[CO2(g)] = 0.8317.) For reaction (2), K = 2.8 x 10-9:
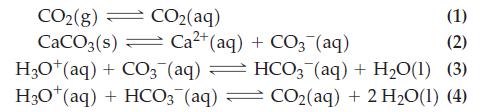
(a) Use the equations above to determine the hydronium ion concentration as a function of [CO2(g)] and [Ca2+].
(b) During preindustrial conditions, we will assume that the equilibrium concentration of [CO2(g)] = 280 ppm and [Ca2+] = 10.24 mM. Calculate the pH of a sample of ocean water.
Transcribed Image Text:
CO2(g) — CO2(aq) (1) CaCO3(s) Ca²+ (aq) + CO3(aq) (2) H3O+ (aq) + CO3(aq) — HCO3(aq) + H₂O(1) (3) H3O+ (aq) + HCO3(aq) CO₂(aq) + 2 H₂O(1) (4) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a Determine the hydronium ion concentration as a function of CO2g and Ca2 From equation 1 we can exp...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
THE DATA PROJECTOR MARKET Alset recognises that mini data projector demand is growing rapidly, although actual numbers are difficult to obtain. The latest figures show the market is $13 billion...
-
A glass of water initially at pH 7.0 is exposed to dry air at sea level at 20C. Calculate the pH of the water when equilibrium is reached between atmospheric CO2 and CO2 dissolved in the water, given...
-
Listed are the equity sections of balance sheets for years 2011 and 2012 as reported by Mountain Air Ski Resorts, Inc. The overall value of stockholders equity has risen from $2,000,000 to...
-
Joses Electronic Repair Shop has budgeted the following time and material for 2014. Joses budgets 5,000 hours of repair time in 2014 and will bill a profit of $5 per labor hour along with a 30%...
-
White Company can invest in one of two projects, TD1 or TD2. Each project requires an initial investment of $101,250 and produces the year-end cash inflows shown in the following table. Required 1....
-
a. Do you agree with the controllers recommendation? Why or why not? b. Formulate an LP model for this problem. c. Create a spreadsheet model for this problem and solve it using Solver. d. What is...
-
3) (5 marks) Given f(x) = -5x+1 and g(x) = 4x - 3, a. Write an equation for (f + g)(x) b. Determine (f+g)(6) c. Determine (fg)(x) d. Determine (f-g)(x) e. Determine the domain of (fg)(x) 1-x 4) Given...
-
In 1922 Donald D. van Slyke (J. Biol. Chem., 52, 525) defined a quantity known as the buffer index: = dc b/ d(pH), where d cb represents the increment of moles of strong base to one liter of the...
-
Avery common buffer agent used in the study of biochemical processes is the weak base TRIS, (HOCH 2 ) 3 CNH 2 , which has a pK b of 5.91 at 25 C. A student is given a sample of the hydrochloride of...
-
Reconsider the axial stiffness data given in Exercise 8. ANOVA output from Minitab follows: Pooled StDev = 32.39. Tukey's pair-wise comparisons Family error rate = 0.0500 Individual error rate =...
-
Bell Media has common stock trading at a price of $74, and a market capitalization of $23 billion. The firm also has preferred stock worth a total of $6 billion, currently trading at $54 per share...
-
how does an investor evaluation of the investment alternatives differ from the evaluation by a company trying to raise funds ? Explain
-
The risk-free rate is 1.88% and the expected return on the market is 9.95%. What is the market risk premium? Round your answer to 2 decimal places.
-
What is the value of sinx cosxdx?
-
Apple expects to pay a dividend of $7 per share next year and expects those dividends to increase at 7% per year. The price of Apple is $185 per share. What is Apples cost of equity?
-
Visit the OECDs Glossary of Statistical Terms at the following web address: stats.oecd.org/glossary/detail.asp?ID=3254 When the market fails there are calls for the government to step in and clean up...
-
Explain the circumstances that could result in a long-term bank loan being shown in a statement of financial position as a current liability.
-
We consider the random process St, which plays a fundamental role in BIack-Scholes analyses: St = S0e[1+Wt] Where Wt is a Wiener process with W0 = 0, is a trend factor, and (Wt Ws) N(0, (I s)),...
-
Show that as n ? ? (a) 1(1 - ).-(1 (b) (1 - )" - . (e) (1-) -1
-
Let the random variable Xn have a binomial distribution: Where each Bi is independent and i distributed according to We can look at X n as the cumulated sum of a series of events that occur over...
-
Fak Industries uses cash basis accounting. The following cash transactions occurred during the month of December: Fak Industries received $10,028 in fees from customers for services performed during...
-
The hotel you work at makes raspberry torte for service at a banquet. The cake recipe used produces enough cake to make 4 tortes which are each cut into 10 portions per torte. It costs $10.92 to...
-
Record the following transactions for a Davis Company: a. May 1, 2022: Sold $3,000 worth of gift cards to customers for cash b. May 10, 2022: Sold goods to customers for $2,000 plus Ontario HST of...

Study smarter with the SolutionInn App


