The solubility of Cl 2 (g) in water is 6.4 g L 1 at 25 C. Some
Question:
The solubility of Cl2(g) in water is 6.4 g L–1 at 25 °C. Some of this chlorine is present as Cl2, and some is found as HOCl or Cl–. For the hydrolysis reaction
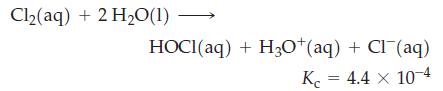
For a saturated solution of Cl2 in water, calculate [Cl2], [HOCl], [H3O+], and [Cl–].
Transcribed Image Text:
Cl₂(aq) + 2 H₂O(1) HOCI(aq) + H3O+(aq) + Cl(aq) Kc = 4.4 x 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
To calculate the concentrations of the various species in a saturated solution of Cl2 in water we ca...View the full answer

Answered By

Dinesh F
I have over 3 years of professional experience as an assignment tutor, and 1 year as a tutor trainee.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The solubility of Cl2 in 100 g of water at STP is 310 cm3. Assume that this quantity of Cl2 is dissolved and equilibrated as follows: (a) If the equilibrium constant for this reaction is 4.7 Ã...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
On April 1, 2014, Briggs Corp. purchases a 24-month property insurance policy for $72,000. The policy is effective immediately. Assume that Briggs prepares adjusting entries only once a year, on...
-
You are an analyst for an investment fund that invests in initial public offerings (IPOs). You are looking at the financial statements of two companies, Clark Company and Durfee Company, that plan to...
-
In 2000, the S&P 500 Index earned -9.1 percent while the T-bill yield was 5.9 percent. Does this mean the market risk premium was negative? Explain.
-
Patty Hayes owned four Personal Seat Licenses (PSLs) at the Cleveland Browns Stadium. Hayess PSLs reserved four seats on the 50-yard line, at the railing, on the north side of the stadium. As the...
-
Anthony Herrera recently fulfilled his long-time dream of opening a gym that offers spinning exercise classes for $5 per person. To differentiate his gym and better serve his clients, Anthony...
-
Following are the ledger balances of Titas Pvt. Ltd. as on the date 31 December, 2022. Prepare the Trial Balance using the following balances. Account Name Tk. Bank Overdraft 40,000 Cash 20,000...
-
Not shown in Figure 22-17 are electrode potential data involving hydrazoic acid. Given that E = -3.09 V for the reduction of HN 3 to N 2 in acidic solution, what is E for the reduction of HN 3 to NH...
-
Write plausible half-equations and a balanced oxidationreduction equation for the disproportionation of XeF 4 to Xe and XeO 3 in aqueous acidic solution. Xe and XeO 3 are produced in a mole ratio,...
-
How would you need to alter an article on reducing pollution from vehicle fumes to appeal to the different readers of these publications?
-
In 2009, Congress and the president set up the Financial Crisis Inquiry Commission to investigate the causes of the financial crisis. At a hearing of the commission in 2010, Robert Rubinwho had...
-
In 2012, the euro-zone countries began the process of increasing the integration of their banking systems by giving the European Central Bank the authority to supervise banks in all member countries....
-
In 2010, an article in the Wall Street Journal observed: In the bond market . . . investors have been flocking to all manner of [bonds] . . . from Treasuries to junk bonds. The attraction: steady...
-
Trader A enters into futures contracts to buy 1 million euros for 1.3 million dollars in three months. Trader B enters in a forward contract to do the same thing. The exchange rate (dollars per euro)...
-
In 2012, as speculation increased that Greece might stop using the euro as its currency, the Wall Street Journal published an article that included this observation: The Continents financial system...
-
Howard Company is 100% owned by Rona. During the current year, Howard sells some land to Rona for $50,000 that had cost Howard $80,000 and that had a fair market value of $100,000. Write a letter to...
-
What are three disadvantages of using the direct write-off method?
-
The following information was taken from the 2009 financial statements of FedEx Corporation, a major global transportation/delivery company. InstructionsAnswer each of the following questions.(a)...
-
The following ratios are available for Tym Inc. Instructions(a) Is Tym??s short-term liquidity improving or deteriorating in 2012? Be specific in your answer, referring to relevant ratios.(b) Do...
-
On March 3, Pitrof Appliances sells $710,000 of its receivables to American Factors Inc. American Factors Inc. assesses a service charge of 4% of the amount of receivables sold. Instructions Prepare...
-
A project has an initial investment of $5 million, and is expected to yield cash inflows of $800,000 per year for the next nine years. The discount rate of the project is 11%. What is the payback...
-
You have been asked to assess the value of synergy in an acquisition of Nuevos Fashion, a children's apparel firm, by Fitch and Spitzer, a general apparel firm. You are supplied with the following...
-
10%This assignment relates to the following Course Learning Requirements:(Insert the applicable ones) Evaluate business financial statements by using meaningful financial ratios and comparative...

Study smarter with the SolutionInn App


