The standard enthalpy of formation of gaseous H 2 O at 298.15 K is -241.82 kJ mol
Question:
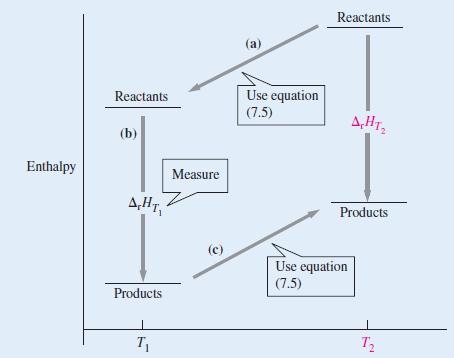
The standard enthalpy of formation of gaseous H2O at 298.15 K is -241.82 kJ mol-1. Using the ideas contained in Figure 7-16, estimate its value at 100.0 °C given the following values of the molar heat capacities at constant pressure: H2O(g): 33.58 J K-1 mol-1; H2(g): 28.84 J K-1 mol-1; O2(g): 29.37 J K-1 mol-1. Assume the heat capacities are independent of temperature.
Figure 7-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





