The standard enthalpy of formation of H 2 O 2 (g) is -136 kJ mol -1 .
Question:
The standard enthalpy of formation of H2O2(g) is -136 kJ mol-1. Use this value, with other appropriate data from the text, to estimate the oxygen-to-oxygen single-bond energy. Compare your result with the value listed in Table 10.3.
Table 10.3
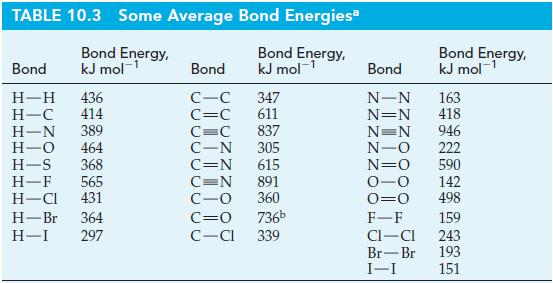
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energies Bond Energy, kJ mol-¹ Bond Energy, kJ mol-¹ Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C C=C 347 611 837 305 C=C C-N C=N 615 C=N 891 C-O 360 C=O 736b C-CI 339 Bond Energy, kJ mol¹ Bond N-N 163 N=N 418 N=N 946 N-O 222 N=O 590 0-0 142 0=0 498 F-F 159 CI-CI 243 Br-Br 193 I-I 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
HOs 12028 HO8 AHAH bonds broken AH bonds formed AH H0H0120...View the full answer

Answered By

Jeff Omollo
As an educator I have had the opportunity to work with students of all ages and backgrounds. Throughout my career, I have developed a teaching style that encourages student engagement and promotes active learning. My education and tutoring skills has enabled me to empower students to become lifelong learners.
5.00+
5+ Reviews
42+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Under Social Security, the family of a worker who dies while fully insured at the time of death has a right to survivors' benefits. True False
-
The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per...
-
The standard enthalpy of formation of the metallocene bis (benzene) chromium was measured in a calorimeter. It was found for the reaction Cr (C6H6)2(s) Cr(s) + 2 C6H6 (g) that Uo (583 K) = +8.0 kJ...
-
1:When developing a marketing strategy for business customers, it is essential to understand the process the business goes through when making a buying decision. Knowledge of business buying behavior...
-
Joses Electronic Repair Shop has budgeted the following time and material for 2014. Joses budgets 5,000 hours of repair time in 2014 and will bill a profit of $5 per labor hour along with a 30%...
-
Locate the centroid y of the composite area, then determine the moment of inertia of this area about the centroidal x'axis. 1 in. 1 in. 5 in. 2 in. -3 in- . 3 in.-
-
Plaintiff grounds manager sued a manufacturer, Monsanto, alleging that herbicide use caused his non-Hodgkins lymphoma. The jury awarded the plaintiff \($39.3\) million in compensatory damages and...
-
Builder Products, Inc., manufactures a caulking compound that goes through three processing stages prior to completion. Information on work in the first department, cooking, is given below for May:...
-
At a price of $340, Vurtego sold 1050 pogo sticks per year. After airing on Shark Tank, demand increased, and Vurtego sold 1950 pogo sticks the following year for $460 a unit. Using the mid-point...
-
Use the VSEPR theory to predict a probable shape of the molecule F 4 SCH 2 , and explain the source of any ambiguities in your prediction.
-
Estimate the enthalpy of formation of HCN using bond energies from Table 10.3, data from elsewhere in the text, and the reaction scheme outlined as follows. Table 10.3 C(g) A,H = ? HCN(g) AH = ? H(g)...
-
Let f : R R be defined by setting f(x) := x if x is rational, and f(x) = 0 if x is irrational. (a) Show that f has a limit at x = 0. (b) Use a sequential argument to show that if c 0, then f does...
-
Unless a contract provides otherwise, it is normally assumed to be a shipment contract. (True/False)
-
What determines who suffers a financial loss if goods are damaged, destroyed, or lost?
-
Does the modification of an agreement subject to the UCC need new consideration to be binding?
-
Kate contracts with Bernardo to transport Bernardos goods to his stores. If this contract is discharged as most contracts are, it will be discharged by a. performance. b. agreement. c. operation of...
-
Unless the parties agree otherwise, title passes at the time and place that the buyer accepts the goods. (True/False)
-
The Digital Guardian Company issues policies that protect clients from downtime costs due to computer system failures. It is very important to process the policies quickly because long cycle times...
-
Distinguish between the work performed by public accountants and the work performed by accountants in commerce and industry and in not-for-profit organisations.
-
Accounting for a byproduct. Washington Oceanic Water (WOW) desalinates and bottles sea water. The desalinated water is in high demand from a large group of environmentally conscious people on the...
-
Alternative methods of joint-cost allocation, product-mix decisions. The Sunshine Oil Company buys crude vegetable oil. Refining this oil results in four products at the splitoft point; A, B, C, and...
-
Comparison of alternative joint-cost-allocation methods, further-processing decision, chocolate products. The Chocolate Factory manufactures and distributes chocolate products. It purchases cocoa...
-
4. Find A transpose; Inverse of A; Transpose of C; Inverse of C 1 1 A=-1 2 -3 0 C = -1 1 using mat lab 5 Find det (A); det (B); det (C); 43 2 1 0 -1 0 0 2-6-7 5 B 0 4 4 C = a
-
Problem 9-2 (Algo) Lower of cost or net realizable value; by product, category, and total inventory [LO9- 1] Ace Hardware Store sells two product categories, tools and paint products. Information...
-
Given a training sample (x(i) = [3,4], y() = 2) for softmax regression of 3 classes. Current parameters for the three classes are = [-7,2,3], 0 = [4,-2,3], 03 [5,4,-2]. (1) (20 points) Find the...

Study smarter with the SolutionInn App


