Estimate the enthalpy of formation of HCN using bond energies from Table 10.3, data from elsewhere in
Question:
Estimate the enthalpy of formation of HCN using bond energies from Table 10.3, data from elsewhere in the text, and the reaction scheme outlined as follows.
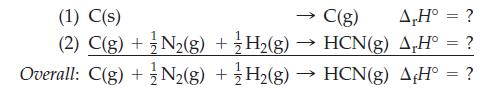
Table 10.3
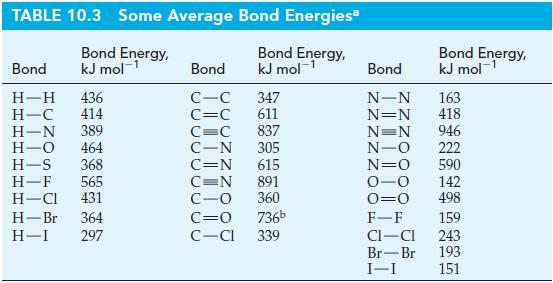
Transcribed Image Text:
C(g) A,H° = ? HCN(g) AH° = ? H₂(g) → HCN(g) AH° = ? (1) C(s) (2) C(g) + N₂(g) + H₂(g) Overall: C(g) + N₂(g) +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
AH717 KJmol C 8 C ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Resonance energy is the difference in energy between a real moleculea resonance hybridand its most important contributing structure. To determine the resonance energy for benzene, we can determine an...
-
Using bond energies from Table, estimate the barrier to rotation around a carboncarbon double bond. To do this, consider what must happen to go from In terms of making and breaking chemical bonds;...
-
The gas-phase reaction shown, between N2 and O2, was run in an apparatus designed to maintain a constant pressure. (a) Write a balanced chemical equation for the reaction depicted and predict whether...
-
Name the types of consumer decision-making processes. List some products you have bought using each type. Have you ever bought a product on impulse? If so, describe the circumstances.
-
Lovell Computer Parts Inc. is in the process of setting a selling price on a new component it has just designed and developed. The following cost estimates for this new component have been provided...
-
Determine the distance y to the centroid of the beam?s cross-sectional area; then find the moment of inertia about the x-axis. 50 mm 50 mm 300 mm 100 mm -200 mm
-
Nereus Montemayor was an employee of VZ Hogs, a company that raises hogs and produces hog feed. VZ Hogs used an extruder manufactured by Sebright Products, Inc. to create hog feed out of discarded...
-
Big Box Store is located in midtown Madison. During the past several years, net income has been declining because of suburban shopping centers. At the end of the companys fiscal year on November 30,...
-
Development economics studies the transformation of emerging nations into more prosperous one and it seeks to understand and shape the country's macro and microeconomics policies in order to lift...
-
The standard enthalpy of formation of H 2 O 2 (g) is -136 kJ mol -1 . Use this value, with other appropriate data from the text, to estimate the oxygen-to-oxygen single-bond energy. Compare your...
-
In certain polar solvents, PCl 5 undergoes an ionization reaction in which a Cl - ion leaves one PCl 5 molecule and attaches itself to another. The products of the ionization are PCl 4 + and PCl 6 -...
-
For each of the problems listed, determine the following quantities for each activity: the earliest start time, latest start time, earliest finish time, latest finish time, and slack time. List the...
-
Madeline Castellotti was the sole shareholder of Whole Pies, Inc., which owns Jons Pizzeria in New York City. Her other assets included an interest in a real estate partnership, a residence on Staten...
-
In a transaction for a sale of goods subject to a shipment contract, when does title pass?
-
Dave contracts with Paul to buy a delivery truck. Dave tells Paul that if the truck is not delivered on Monday, he will lose $12,000 in business. Paul does not deliver the truck on Monday. Dave is...
-
Privity of contract is required to hold a manufacturer liable in a product liability action based on negligence. (True/False)
-
If a contract calls for the sale or lease of goods that are already in existence, when does identification take place?
-
Representatives of the Patriot Insurance Company take medical information over the telephone from prospective policy applicants prior to a visit to the applicants place of residence by a registered...
-
Economic feasibility is an important guideline in designing cost accounting systems. Do you agree? Explain.
-
Joint-cost allocation, process further or sell. (CMA, adapted) Sonimad Sawmill, Inc. (381), purchases logs from independent timber contractors and processes the logs into three types of lumber...
-
Joint-cost allocation. Elsie Dairy Products Corp buys one input full-cream milk, and refines it in a churning process. From each gallon of milk Elsie produces two cups (one pound) of butter and two...
-
Further processing decision (continuation of 18-30). Elsie has decided that buttermilk may sell better if it was marketed for baking and sold in pints. This would involve additional packaging at an...
-
Given the CFG G as follows: EE+T T T-TXF | F Give the derivations for each string. (a) a (c) a+a F(E) a (b) a+a+a (d) ((a))
-
7. Consider the following GNFA. Find an equivalent GNFA with only two states. S b ab+ba 9o ab
-
Write a program that can be used as a math tutor for a young student. The program should display two random numbers to be added, such as 247 +129 The program should then pause while the student works...

Study smarter with the SolutionInn App


