Use data from Appendix D (Table D-2) to calculate a value of E for the reduction of
Question:
Use data from Appendix D (Table D-2) to calculate a value of E° for the reduction of Li+(aq) to Li(s), and compare your result with the value listed in Table 21.2.
Table D-2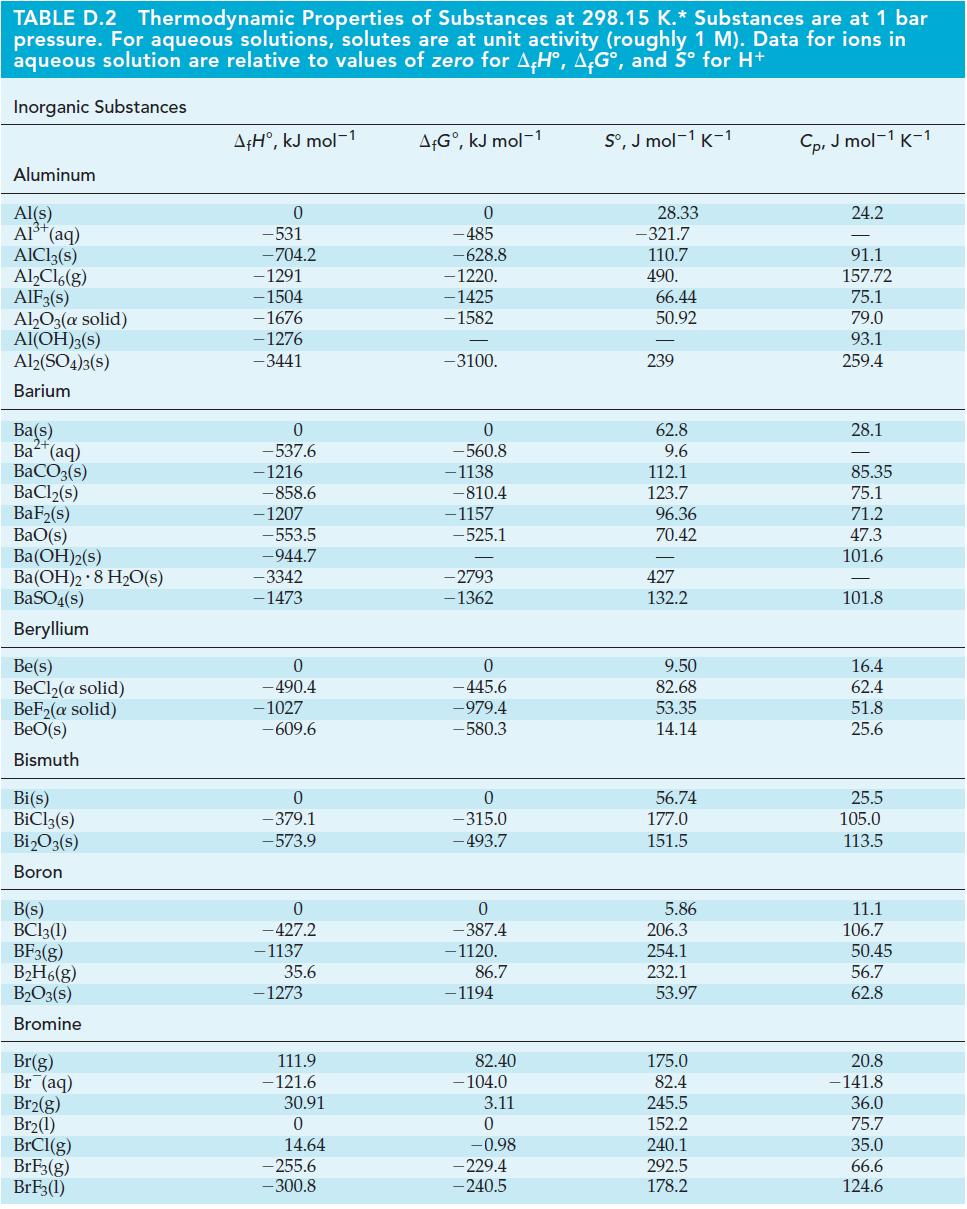
Table 21.2
Transcribed Image Text:
TABLE D.2 Thermodynamic Properties of Substances at 298.15 K.* Substances are at 1 bar pressure. For aqueous solutions, solutes are at unit activity (roughly 1 M). Data for ions in aqueous solution are relative to values of zero for AH, AG°, and Sº for H+ Inorganic Substances Aluminum Al(s) Al³+ (aq) AIC13(s) Al₂Cl6(g) AIF 3(s) Al₂O3(a solid) Al(OH)3(s) Al2(SO4)3(S) Barium Ba(s) Ba (aq) BaCO3(s) BaCl₂(s) BaF₂(s) BaO(s) Ba(OH)2(s) Ba(OH)2 8 H₂O(s) BaSO4(s) Beryllium Be(s) BeCl₂(a solid) BeF₂(a solid) BeO(s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Boron B(s) BC13(1) BF3(g) B₂H6(g) B₂O3(s) Bromine Br(g) Br (aq) Br2(g) Br₂(1) BrCl(g) BrF3(g) BrF3(1) A+H°, kJ mol-1 0 -531 -704.2 -1291 -1504 -1676 -1276 -3441 0 -537.6 - 1216 -858.6 -1207 -553.5 -944.7 -3342 -1473 0 - 490.4 -1027 -609.6 0 -379.1 -573.9 0 -427.2 - 1137 35.6 - 1273 111.9 -121.6 30.91 0 14.64 -255.6 -300.8 A+Gº, kJ mol-1 0 -485 -628.8 -1220. -1425 - 1582 -3100. 0 -560.8 -1138 -810.4 -1157 -525.1 -2793 -1362 0 -445.6 -979.4 -580.3 0 -315.0 - 493.7 0 -387.4 -1120. 86.7 - 1194 82.40 -104.0 3.11 0 -0.98 -229.4 -240.5 Sº, J mol-1 K-1 28.33 -321.7 110.7 490. 66.44 50.92 239 62.8 9.6 112.1 123.7 96.36 70.42 427 132.2 9.50 82.68 53.35 14.14 56.74 177.0 151.5 5.86 206.3 254.1 232.1 53.97 175.0 82.4 245.5 152.2 240.1 292.5 178.2 Cp, J mol-1 K-1 24.2 91.1 157.72 75.1 79.0 93.1 259.4 28.1 85.35 75.1 71.2 47.3 101.6 101.8 16.4 62.4 51.8 25.6 25.5 105.0 113.5 11.1 106.7 50.45 56.7 62.8 20.8 - 141.8 36.0 75.7 35.0 66.6 124.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Lile Lis E 3050v STP AGnFE 196500 3050294325Jmol ...View the full answer

Answered By

Joram mutua
I am that writer who gives his best for my student/client. Anything i do, i give my best. I have tutored for the last five years and non of my student has ever failed, they all come back thanking me for the best grades. I have a degree in economics, but i have written academic papers for various disciplines due to top-notch research Skills.In additional, I am a professional copywriter and proofreader.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In one type of Breathalyzer (alcohol meter), the quantity of ethanol in a sample is related to the amount of electric current produced by an ethanoloxygen fuel cell. Use data from Table 19.1 and...
-
Use the data in Appendix 3 to calculate the equilibrium constant for the reaction Agl(s) Ag+(aq) + I2(aq) at 25C. Compare your result with the Ksp value in Table 16.2.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Recent trends in recruiting rely on social capital by ______. Multiple choice question. making use of structural holes between groups in a social network where there are few relationships bridging...
-
Silva and Juanita Rodriquez are the owners of Year-Round Landscape, Inc., a small landscape and yard service business in southern California. The business is three years old and has grown...
-
In the aftermath of the financial crisis of 2007-2009, there have been calls to re-instate the separation of commercial and investment banking activities that were removed with the repeal of the...
-
Refer to the information in Exercise 17-4. Required 1. Compute a departmental overhead rate for the molding department based on machine hours and a department overhead rate for the trimming...
-
Balance Sheet Classification of Various Liabilities how would each of the following items be reported on the balance sheet? (a) Accrued vacation pay. (b) Estimated taxes payable. (c) Service...
-
1. Show that the Ramsey number R(m, n) = R(n, m), for all m 2, n 2 positive integers. 2. Show that the Ramsey number R(3, 4) 10. 3. Show that the Ramsey number R(4, 4) < 20.
-
The electrolysis of 0.250 L of 0.220 M MgCl 2 is conducted until 104 mL of gas (a mixture of H 2 and water vapor) is collected at 23 C and 748 mmHg. Will Mg(OH) 2 (s) precipitate if electrolysis is...
-
Concerning the thermite reaction, (a) Use data from Appendix D to calculate r H at 298 K for the reaction below. (b) Write an equation for the reaction when MnO 2 (s) is substituted for Fe 2 O 3...
-
Julio and Milania are owners of Falcons Corporation, an S corporation. They each own 50 percent of Falcons Corporation. In year 1, Julio and Milani each received distributions of $15,000 from Falcons...
-
In a Forbes magazine article called Worst Words to Say at Work, business consultant and psychotherapist Linda Durre listed nine words or phrases that show someone is not confident.9 These phrases,...
-
Choose a business report to evaluate. Answer the following questions related to it: A. How effectively does the report tell a story? B. How effectively are headings used? C. How effectively are...
-
Think about a recent presentation you attended that you found effective. In three to five paragraphs, describe why it was effective. Include the following aspects in your analysis, referring to...
-
Watch a YouTube video (or some other type of multimedia example) that shows a realistic mock interview. Put yourself in the place of the interviewee and use the KE YS process to analyze his or her...
-
Many times in the workplace, you will create a written report and provide an oral presentation as well. Select a written report and develop a set of electronic slides that could be used to present...
-
During the year, Grace, Inc., has total sales of $800,000. Based on total sales, the corporation estimates that its bad debts for the year are 2% of sales. As a result, the corporation deducts...
-
QUESTION 9 HC-O-C-R R-C-O-CH HC-O-P-O-CH-CH-NH3* O || O a. Phosphatidic acid, Serine O b. Lysophosphatidic acid, Serine, Free FA O c. Lysophosphatidylserine, Free FA O d. 2 Free FAs, Serine, Glycerol...
-
Some of the largest business frauds ever perpetrated have involved the misstatement of inventory. Two classics were at Leslie Fay Cos, and McKesson Corporation. Instructions There is considerable...
-
If your school has a subscription to the FASB Codification, prepare responses to the following. (a) The primary basis for accounting for inventories is cost. How is cost defined in the Codification?...
-
Briefly describe some of the similarities and differences between GAAP and IFRS with respect to the accounting for inventories.
-
Why is knowing the market is very important when investigating a business one show interest in buying?
-
Discuss the requirements to apply OLS estimation when using differenced datax.
-
Permata Sdn Bhd manufactures and sells its single Product X through manufacturer agents. The agents are paid a commission of 20% of the selling price. In the coming financial year, the sales agents'...

Study smarter with the SolutionInn App


