Use data from Appendix D (Table D-2) to estimate the minimum voltage required to electrolyze Al 2
Question:
Use data from Appendix D (Table D-2) to estimate the minimum voltage required to electrolyze Al2O3 in the Hall-Héroult process, reaction (21.25). Use ΔfG°[Al2O3(l)] = –1520 kJ mol–1. Show that the oxidation of the graphite anode to CO2(g) permits the electrolysis to occur at a lower voltage than if the electrolysis reaction were Al2O3(l) → 2 Al(l) + 3/2O2(g).
Table D-2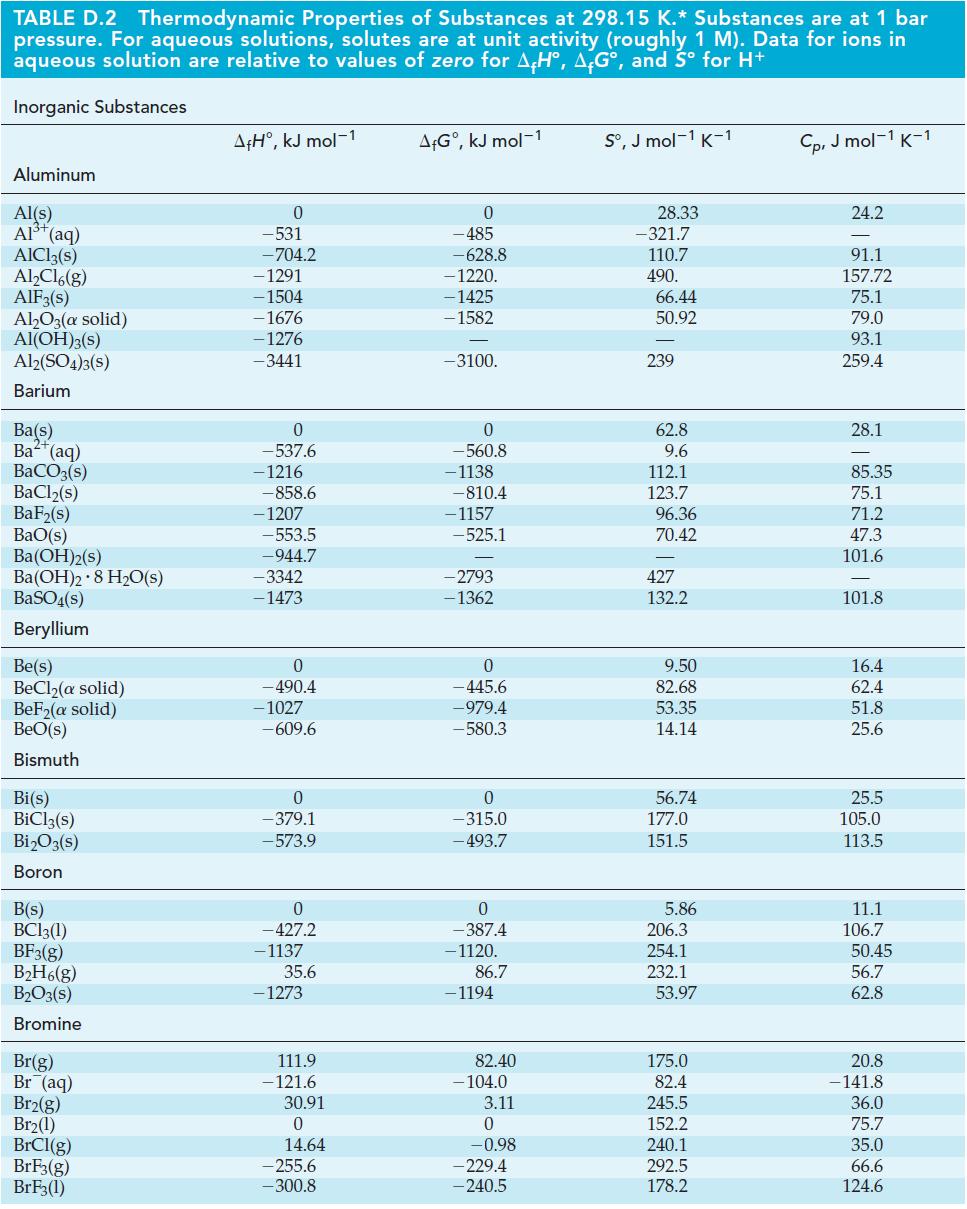
Reaction (21.25)
![]()
Transcribed Image Text:
TABLE D.2 Thermodynamic Properties of Substances at 298.15 K.* Substances are at 1 bar pressure. For aqueous solutions, solutes are at unit activity (roughly 1 M). Data for ions in aqueous solution are relative to values of zero for AH, AG°, and Sº for H+ Inorganic Substances Aluminum Al(s) Al³+ (aq) AlCl3(s) Al₂Cl6(g) AlF3(s) Al₂O3(a solid) Al(OH)3(s) Al2(SO4)3(S) Barium Ba(s) Ba²+ (aq) BaCO3(s) BaCl₂(s) BaF₂(s) BaO(s) BA(OH)2(s) Ba(OH)2 8 H₂O(s) BaSO4(s) Beryllium Be(s) BeCl₂(a solid) BeF₂(a solid) BeO(s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Boron B(s) BC13(1) BF3(g) B₂H6(g) B₂O3(s) Bromine Br(g) Br (aq) Br2(g) Br₂(1) BrCl(g) BrF3(g) BrF3(1) A+H°, kJ mol-1 0 -531 -704.2 -1291 -1504 -1676 -1276 -3441 0 -537.6 - 1216 -858.6 -1207 -553.5 -944.7 -3342 -1473 0 - 490.4 -1027 -609.6 0 -379.1 -573.9 0 -427.2 - 1137 35.6 - 1273 111.9 -121.6 30.91 0 14.64 -255.6 -300.8 A+Gº, kJ mol-1 0 -485 -628.8 -1220. -1425 - 1582 -3100. 0 -560.8 -1138 -810.4 -1157 -525.1 -2793 -1362 0 -445.6 -979.4 -580.3 0 -315.0 - 493.7 0 -387.4 -1120. 86.7 - 1194 82.40 -104.0 3.11 0 -0.98 -229.4 -240.5 Sº, J mol-1 K-1 28.33 -321.7 110.7 490. 66.44 50.92 239 62.8 9.6 112.1 123.7 96.36 70.42 427 132.2 9.50 82.68 53.35 14.14 56.74 177.0 151.5 5.86 206.3 254.1 232.1 53.97 175.0 82.4 245.5 152.2 240.1 292.5 178.2 Cp, J mol-1 K-1 24.2 91.1 157.72 75.1 79.0 93.1 259.4 28.1 85.35 75.1 71.2 47.3 101.6 101.8 16.4 62.4 51.8 25.6 25.5 105.0 113.5 11.1 106.7 50.45 56.7 62.8 20.8 - 141.8 36.0 75.7 35.0 66.6 124.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In Example 13-3, we dealt with vap H and vap S for water at 100 C. (a) Use data from Appendix D to determine values for these two quantities at 25 C. (b) From your knowledge of the structure of...
-
The overall reaction for the electrolytic production of aluminum by means of the Hall process may be represented as Al2O3 (s) + 3C (s) 2Al(l) + 3CO(g) At 1000C, the standard free-energy change for...
-
Evaluate each expression if possible. -V0.49
-
What is the key criterion for using the equity method of accounting for equity securities?
-
Briefly discuss OLAP architectural styles with and without data marts.
-
Plaintiffs purchased stock warrants (rights to purchase) for blocks of Osborne Computer Corp., the manufacturer of the first mass-market portable personal computer. Because of inability to produce a...
-
Martin Shoes, Inc. manufactures and distributes orthopedic footwear. To sell its products, the marketing department requires sales personnel to call on the shoe retailers within their assigned...
-
1. (10) A $10 000 bond was issued on January 1st 2015 with a coupon rate of 9.7% and a redemption date of January 1st 2025. What is the purchase price of the bond on January 1st, 2022 when the yield...
-
The nitrates MNO 3 (M = Na, K, Rb, Cs) decompose to the nitrites (MNO 2 ) on heating; while in contrast, LiNO 3 decomposes to Li 2 O. Suggest a reason for this difference in behavior. Write balanced...
-
In a manner similar to that outlined on page 1022, (a) Write equations to represent the reaction of (CH 3 ) 3 SiCl with water, followed by the elimination of H 2 O from the resulting silanol...
-
From Exercises 101 and 102, the nonreal complex solutions of the equation are 1 + 5i and 1 - 5i. What do we call two complex numbers a + bi and a - bi? Data from in Exercises 101 Show that 1 + 5i is...
-
(1) A 1200 kg racecar goes at a constant speed of 120 km/h round a curved section of track that has a radius of 60 m. (i). What is the speed of the car in m/s? (ii). What is the car's centripetal...
-
a program that calculates incentive bonus pay for employees on a sales team. An employee earns $500 weekly as base pay, there is a bonus scale for various levels of sale quota each week. If the...
-
I need JAVA CODE TO BE MODIFIED TO ACCOMPLISH AS PER THE INSTRUCTION BELOW: Employee Class: The Employee information you must track is as follows: ID (This is generated randomly) Name Gender Job...
-
A hollow steel ball of mass kg is suspended from a spring. This stretches the spring m. The ball is started in motion from the equilibrium position with a downward velocity of 0.3 meters per second....
-
You must use GridBagLayout for this assignment. Must have the application that displays a menu of items on the left hand side and displays the total of all the items selected on the right. The...
-
Explain why someone might think this statement is true: The parole evidence rule makes people more careful about writing contracts.
-
Is the modified 5-question approach to ethical decision making superior to the modified moral standards or modified Past in approach?
-
Suppose you held a diversified portfolio consisting of a $7,500 investment in each of 20 different common stocks. The portfolios beta is 1.12. Now suppose you decided to sell one of the stocks in...
-
Industries (HRI) has a beta of 1.8, while LR Industries (LRI) beta is 0.6. The risk-free rate is 6%, and the required rate of return on an average stock is 13%. The expected rate of inflation built...
-
You have been managing a $5 million portfolio that has a beta of 1.25 and a required rate of return of 12%. The current risk-free rate is 5.25%. Assume that you receive another $500,000. If you...
-
2. Write a Java program that will use a two-dimensional array and modularity to solve the following tasks: 1. Create a method to generate a 2-dimensional array (random numbers, range 0 - 500). The...
-
The table below represents the regression output for a company estimating the cost of material handling. The company has identified that any variable cost related to this activity is driven by the...
-
Write a program named TwoDArray with a two-dimensional integer array. The program asks user to enter number of rows and columns for the array, and then uses an object of class Random to fill out the...

Study smarter with the SolutionInn App


