Use data from Table 10.3, but without performing detailed calculations, determine whether each of the following reactions
Question:
Use data from Table 10.3, but without performing detailed calculations, determine whether each of the following reactions is exothermic or endothermic.
![]()
Table 10.3
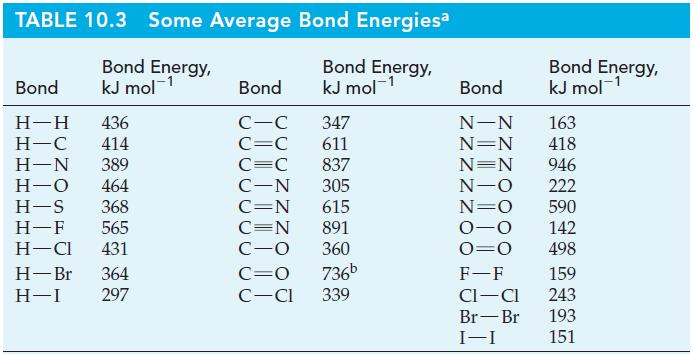
Transcribed Image Text:
(a) CH4(g) + I(g) (b) H₂(g) + I2(g) CH3(g) + HI(g) 2 HI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
a CHg CH3 HIg AH for CH414 KJmol AH for I000 KJmol AH for HI 297 KJmol AH ...View the full answer

Answered By

Allan Olal
I have vast tutoring experience of more than 8 years and my primary objective as a tutor is to ensure that a student achieves their academic goals.
4.70+
78+ Reviews
409+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Determine whether each of the following reactions proceeds via an S N 1 or S N 2 mechanism and then draw the product(s) of the reaction: (a) (b) (c) (d) (e) (f) .? , Br cP HMPA
-
BACKGROUND You are an information analyst working for NEE. The company president has asked you to prepare a Quantitative analysis of financial, sales, and operations data to help determine which...
-
When Ralph Lauren makes shirts to a customers exact preferences, what utility is provided?
-
Paige Corporation makes a mechanical stuffed alligator that sings the Martian national anthem. The following information is available for Paige Corporations anticipated annual volume of 500,000...
-
Modigliani and Miller (MM) on the one hand and Gordon and Lintner (GL) on the other hand have expressed strong views regarding the effect of dividend policy on a firms cost of capital and value. a....
-
Leicht Transfer & Storage provides warehousing services and often purchases pallets from Pallet Central. The companies followed a standard practice for documenting these transactions in which Pallet...
-
Data pertaining to the current position of Forte Company are as follows: Cash ....................$412,500 Marketable securities.............. 187,500 Accounts and notes receivable (net)............
-
6. MAC =9 The use of energy is one of the major causes of pollution and greenhouse gases that lead to climate change. It is desirable to reduce the emissions from energy use. Suppose there are two...
-
One of the isomers of chloromethanol has the formula ClCH 2 OH. Sketch, by using the dash and wedge symbolism, this isomer of chloromethanol, and indicate the various bond angles.
-
Sketch, by using the dash and wedge symbolism, the H 2 NCH 2 CHO molecule, and indicate the various bond angles.
-
Why is it risky to rely on wholesale deposits for funding?
-
Economic forecasters predict that the rate of inflation in Singapore will be at 3% over the next few decades. The following table shows the nominal interest paid on Treasury securities having...
-
The Akva Group ASA equity accounts for 20082010 are given below. Explain what each item is and how it changed over time. EQUITY Paid-in capital Share capital Share premium reserve Other paid-in...
-
T&T, Inc., designs a product that is safe when used properly. Bob uses the product in an unforeseeable, improper way. If Bob sues T&T, the manufacturer will likely be held a. liable for negligence or...
-
(Budgetary Entries General Ledger) The city of Cherokee Hill adopted its fiscal year 20X8 General Fund budget, using the modified accrual basis of accounting, on January 1, 20X8. Budgeted revenues...
-
The equity accounts for ABC plc are as follows: a) What are the ordinary share and total equity values for the equity account? (b) The company has decided to issue 5,000 shares of equity at a price...
-
Consider your last (or current) job. a. What activities did you perform? b. Who were your customers (internal and external), and how did you interact with them? c. How could you measure the customer...
-
Catalytic hydrogenation of naphthalene over PdC results in rapid addition of 2 moles of H 2 . Propose a structure for this product.
-
Theory of constraints, throughput contribution, quality, relevant costs. Aardee Industries manufactures pharmaceutical products in two departments: Mixing and Tablet-Making. Additional information on...
-
Theory of constraints, contribution margin , sensitivity analysis. Low Tech Toys (LTT) produces dolls in two processes: molding and assembly. LTT is currently producing two models: Chatty Chelsey and...
-
Quality improvement, Pareto diagram, cause-and-effect diagram. The Murray Corporation manufactures, sells, and installs photocopying machines. Murray has placed heavy emphasis on reducing defects and...
-
Assuming that TV Guide decided that their upside was insufficient and wanted to double their royalty from 10% to 20%, and also assuming that fixed costs were to include the initial production run of...
-
In the production and sale of 2,000 units of a single product of the company, the fixed cost amounts to 50,000$ and the variable cost amounts to 80,000$. If production increases to 2,500 units, what...
-
Heads-Up Company sold 7,000 scooter helmets at $96.00 each this fiscal year. Unit variable costs were $60.00 (includes direct material, direct labor, variable manufacturing overhead, and variable...

Study smarter with the SolutionInn App


