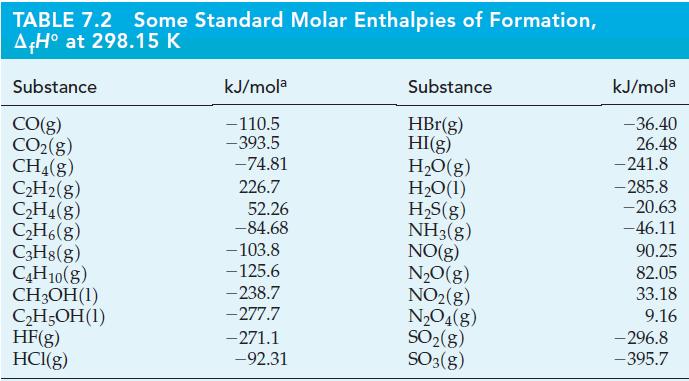
Use data from Table 7.2 to calculate the standard enthalpies of combustion of the four alkane hydrocarbons
Question:
Use data from Table 7.2 to calculate the standard enthalpies of combustion of the four alkane hydrocarbons listed there.
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, A+H° at 298.15 K Substance CO(g) CO₂(g) CH4(g) C₂H₂(g) C₂H4(g) C₂H6(g) C3H8(g) C4H10(g) CH3OH(1) C₂H5OH (1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The standard enthalpy of combustion for an alkane hydrocarbon can be calc using the enthalpies of fo...View the full answer

Answered By

Nicole omwa
Being a highly skilled tutor with at least 5 years of tutoring experience in different areas, I learned how to help diverse learners in writing drafts of research papers, actual research papers and locate credible sources. My assurance is built upon my varied knowledge of a variety of subjects. Furthermore, my involvement and interaction with numerous learners of all levels has allowed me to understand my clients' specific demands. Ultimately, this has aided me in being a better coach to learners to better their grades. Essentially, my responsibilities as a tutor would include:
Teaching abilities that assist pupils in enhancing their academic performance
Personal interaction with learners to make them understand abstract concepts
Inducing new skills and knowledge into their academic journeys
Fostering individual reflection, and independent and critical thinking
Editing and proofreading
Because I am constantly available to respond to your queries, you may decide to rely on me whenever you require my assistance. As an assurance, my knowledge skills and expertise enable me to quickly assist learners with different academic challenges in areas with difficulty in understanding. Ultimately, I believe that I am a reliable tutor concerned about my learner's needs and interests to solve their urgent projects. My purpose is always to assist them in comprehending abstract schoolwork and mastering their subjects. I also understand that plagiarism is a severe offense and has serious ramifications. Owing to this, I always make it a point to educate learners on the numerous strategies to have uniquely unique solutions. I am familiar with the following formatting styles:
MLA
APA
Harvard
Chicago
IEEE
Communication is always the key in every interaction with my learners. Hence, I provide timely communication about the progress of assigned projects. As a result, I make sure that I maintain excellent communication with all of my clients. I can engage with all of my customers more effectively, assisting them with their unique academic demands. Furthermore, I attempt to establish a solid working relationship with my leaners I have exceptional abilities in the below areas;
Sociology
History
Nursing
Psychology
Literature
Health and Medicine
Chemistry
Biology
Management
Marketing
Business
Earth Science
Environmental Studies
Education
Being a teacher who aces in diverse fields, I provide various academic tasks, which include;
Academic Reports
Movie Reviews
Literature Reviews
Annotated bibliographies
Lab reports
Discussion posts
Dissertations
Case study analyses
Research proposals
Argumentative Essays
I guarantee you high-quality Papers!!!!!
5.00+
17+ Reviews
32+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Based on the results of Exercise 71, which alkane evolves the greatest amount of heat upon combustion on (a) A per mole basis and (b) A per gram basis? Which is the most desirable alkane from the...
-
(A) Use data from Table 7.2 to calculate the standard enthalpy of combustion of ethanol, C 2 H 5 OH(l), at 298.15 K. (B) Calculate the standard enthalpy of combustion at 298.15 K per mole of a...
-
The standard enthalpies of formation of gaseous propyne (C3H4), propylene (C3H6), and propane (C3H8) are + 185.4, +20.4, and -103.8 kJ/mol, respectively. (a) Calculate the heat evolved per mole on...
-
Sunblessed Juice Company sells bags of oranges and cartons of orange juice. Sunblessed grades oranges on a scale of 1 (poor) to 10 (excellent). At present, Sunblessed has 100,000 pounds of grade 9...
-
How can a person tell whether an entry to an expense account is payment for a legitimate expenditure or a means of concealing a theft of cash?
-
The partnership of Ali, Bev, and Cal became insolvent during 2016, and the partnership ledger shows the following balances after all partnership assets have been converted into cash and all available...
-
Dogs The number of dogs per household in a small town Dogs 0 1 2 3 4 5 Households 1491 425 168 48 29 14
-
During 2012, Doxey Corporation had the following transactions and related events: Jan. 15 Issued 6,500 shares of common stock at par ($16 per share), bringing the total number of shares outstanding...
-
Wildhorse Co.reported cost of goods sold as follows. Beginning inventory Cost of goods purchased Cost of goods available for sale Less: Ending inventory Cost of goods sold 2022 $ 28,120 176,350...
-
The structures of the NH 3 and NF 3 molecules are similar, yet the dipole moment for the NH 3 molecule is rather large (1.47 debye) and that of the NF 3 molecule is rather small (0.24 debye). Provide...
-
Supply an appropriate name for each of the following: (a) HPO 4 2 ; (b) Ca 2 P 2 O 7 ; (c) H 6 P 4 O 13 .
-
A competing model of health care costs to the model given in the previous exercise is the uniform price elasticity model. Using the definition of elasticity of demand from Section 6.3, this model...
-
Some people jump at the chance to be a change agent while others run from the role. Why is this the case? What are some characteristics of successful change agentry? These factors should refer to the...
-
An S corporation with $50,000 of earnings and profits owns rental real estate and has interest income producing investments. For the last two years, 50% of its gross receipts came from passive...
-
How has Biden Lowered premiums and out of pocket costs for millions of Americans?
-
Product costs using activity rates Body-Solid Inc. manufactures elliptical exercise machines and treadmills. The products are prouced in its Fabrication and Assembly production departments. In...
-
How to start an essay on the multigenerational workforce and your experiences working with each of the generations. Begin your essay with an introduction that outlines the current generations in the...
-
Let f, g: Bn B. Define the relation "
-
Parkin Industries, a U.S. company, acquired a wholly-owned subsidiary, located in Italy, at the beginning of the current year, for 200,000. The subsidiary's functional currency is the euro. The...
-
Jane Noonan is the bookkeeper for Wilson Company, Inc. Jane has been trying to get the company??s balance sheet to balance. She finally got it to balance, but she still isn??t sure that it is...
-
Rules governing the investment practices of individual certified public accountants prohibit them from investing in the stock of a company that their firm audits. The Securities and Exchange...
-
Some people are tempted to make their finances look worse to get financial aid. Companies sometimes also manage their financial numbers in order to accomplish certain goals. Earnings management is...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


