(A) Use data from Table 7.2 to calculate the standard enthalpy of combustion of ethanol, C 2...
Question:
(A) Use data from Table 7.2 to calculate the standard enthalpy of combustion of ethanol, C2H5OH(l), at 298.15 K.
(B) Calculate the standard enthalpy of combustion at 298.15 K per mole of a gaseous fuel that contains C3H8 and C4H10 in the mole fractions 0.62 and 0.38, respectively.
Table 7.2
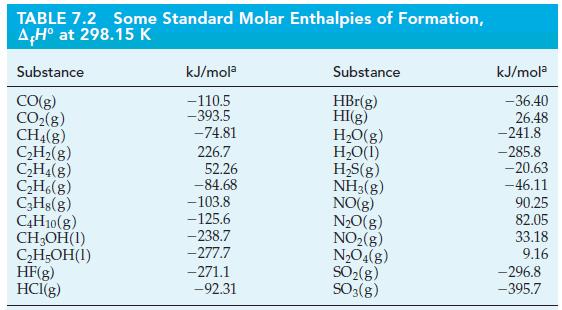
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, A,Hº at 298.15 K Substance CO(g) CO₂(g) CH4(g) C₂H₂(g) C₂H4(8) C₂H6(g) C₂H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola - 36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
1 Answer to Part A The balanced equation for the combustion of ethanol is C2H5OHl 3O2g 2CO2g 3H2O...View the full answer

Answered By

Morgan Njeri
Very Versatile especially in expressing Ideas in writings.
Passionate on my technical knowledge delivery.
Able to multitask and able to perform under pressure by handling multiple challenges that require time sensitive solution.
Writting articles and video editing.
Revise written materials to meet personal standards and satisfy clients demand.
Help Online Students with their course work.
4.90+
12+ Reviews
38+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Ethanol (C2H5OH) has been proposed as an alternative fuel. Calculate the standard enthalpy of combustion per gram of liquid ethanol.
-
The standard enthalpy of combustion of solid urea (CO (NH2)2) is -632 kl mol-1 at 298 K and its standard molar entropy is 104.60 J K-1 mol-1, Calculate the standard Gibbs energy of formation of urea...
-
The molar heat capacity of ethane is represented in the temperature range 298 K to 400 K by the empirical expression Cp,m/ (J K-1 mol-1) = 14.73 + 0.1272(T/K). The corresponding expressions for C(s)...
-
What is the wavelength of light if its frequency is 1.009 106 Hz?
-
1. Which two short-term liquidity ratios measure how frequently a company collects its accounts? 2. What measure reflects the difference between current assets and current liabilities? 3. Which two...
-
The business risk of a particular company is most accurately measured by the companys: A. debt-to-equity ratio. B. efficiency in using assets to generate sales. C. operating leverage and level of...
-
A construction contract has the following language: It is the responsibility of the contractor to inspect and become familiar with the Project and to acquaint itself thoroughly with all conditions...
-
Tool Co. is a medium-sized company that buys copper rod and plastic materials to produce insulated copper wiring. Tool Co. operates out of a single building of about 500,000 square feet that includes...
-
Consider two oppositely charged atoms with charges of +1 and -1 units, respectively. The two atoms interact with each other through electrostatic (Coulombic) and van der Waals forces, and reach an...
-
The AGRI Venture: An Integrated Marketing Communications Program. Chapter 16 states that there are three major forms of cooperative advertising: horizontal, ingredient-sponsored and vertical. Discuss...
-
(A) The overall reaction that occurs in photosynthesis in plants is Determine the standard enthalpy of formation of glucose, C 6 H 12 O 6 (s), at 298.15 K. (B) A handbook lists the standard enthalpy...
-
(A) The standard enthalpy of formation for the amino acid leucine, C 6 H 13 O 2 N(s), is -637.3 kJ/mol. Write the chemical equation to which this value applies. (B) How is r H for the following...
-
Solve each system. 5.5x2.5y + 1.62 = 11.83 2.2x + 5.0y 0.12 = -5.97 - 3.3x - 7.5y + 3.2z = 21.25
-
solve the following [(3 (3x5 - 5x) dx
-
Which two factors contributed to the stock market crash?
-
How do outcomes play out for factor owners when trade opens? (E.g. What happens to labor earnings for the labor-abundant country?) Address capital and labor income in both settings, when a given...
-
Y = Y f = 100 D = 10 +10 E ? P*/P + 0.4( Y?T ) + I+G I = 15 G = 30 T = 25 P* = 1.0 M d /P = 0.01 Y/R M S = 40 R* = 0.02 Calculate the long-run equilibrium price level ( P ) and the long-run...
-
Employment is a relationship between an employee and an employer with expectations by each that the responsibilities of the other will be fulfilled. Sometimes those expectations are not achieved,...
-
Daisy Luna opened a small motorcycle repair shop, Luna Cycle Repair, on January 2, 2014. The shop also sells a limited number of motorcycle parts. In January 2015, Luna realized she had never filed...
-
When an electric field is applied to a shallow bath of vegetable oil, why do tiny bits of thread floating in the oil align with the field like compasses in a magnetic field?
-
Ratio Computations and Effect of Transactions Presented below is information related to Leland Inc. (a) Compute the following ratios or relationships of Leland Inc. Assume that the ending account...
-
Current Liability Entries and Adjustments Described below are certain transactions of Edward son Corporation. The company uses the periodic inventory system. 1. On February 2, the corporation...
-
Liability Entries and Adjustments Listed below are selected transactions of Schultz Department Store for the current year ending December 31. 1. On December 5, the store received $500 from the...
-
The biggest influencer in obesity is an individual's lifestyle choices. Obesity is viewed as preventable (Edberg, 2020). There are many factors that play a role in a person's health, but I will make...
-
You are given the following information for Golden Fleece Financial: Long-term debt outstanding: Current yield to maturity (debt) $370,000 10% Number of shares of common stocki 13,500 Price per...
-
Even though most corporate bonds in the United States make coupon payments semiannually, bonds issued elsewhere often have annual coupon payments. Suppose a German company issues a bond with a par...

Study smarter with the SolutionInn App


