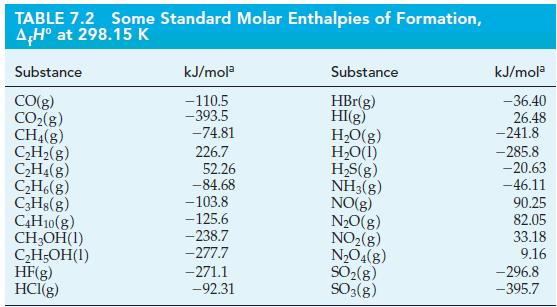
Use data from Table 7.2 to calculate the volume of butane, C 4 H 10 (g), measured
Question:
Use data from Table 7.2 to calculate the volume of butane, C4H10(g), measured at 24.6 °C and 756 mmHg, that must be burned to liberate 5.00 x 104 kJ of heat.
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, AH° at 298.15 K Substance CO(g) CO₂(g) CH4(8) C₂H₂(g) C₂H4(g) C₂H6(8) C3H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCI(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To calculate the amount of butane C4H10 that must be burned to liberate 500 104 kJ of heat we need t...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the mass of octane, C8H18(l), that must be burned in air to evolve the same quantity of energy as produced by the fusion of 1.0 g of hydrogen in the following fusion reaction: Assume that...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Carol Harris, Ph.D, CPA, is a single taxpayer and she lives at 674 Yankee Street, Durham, NC 27409. Her Social Security number is 793-52-4335. Carol is an Associate Professor of Accounting at a local...
-
is an acute angle such that tan() =1/3. What is the value of 1010. (sin + cos)? 3.
-
Fast Ink produces ink-jet printers for personal computers. It received an order for 400 printers from a customer. The following information is available for this order. Process time . . . . . . . . ....
-
I. Dodgem's statement of financial position as at 31 December 2015 was as follows: An opportunity had arisen for Dodgem to acquire the business of A. Swing who is retiring. Dodgem agreed to take over...
-
Juliette Shulof Furs (JSF) was a New York corporation that had been in the fur-dealing business for 15 years. George Shulof, an officer of JSF, attended two auctions conducted by Finnish Fur Sales...
-
Wiset Company completes these transactions during April of the current year (the terms of all its credit sales are 2/10, n/30). Apr. 2 Purchased $ 14,300 of merchandise on credit from Noth Company,...
-
Ethel has an existing loan which she wishes to refinance to obtain a lower rate. Her home has appraised for 352,000. She has requested a new loan in the amount of 264,000 to pay off her old loan and...
-
Diana and Ryan Workman were married on January 1 of last year. Diana has an eight-year-old son, Jorge, from her previous marriage. Ryan works as a computer programmer at Datafile Inc. (DI) earning a...
-
The decomposition of limestone, CaCO 3 (s), into quicklime, CaO(s), and CO 2 (g) is carried out in a gas-fired kiln. Use data from Appendix D to determine how much heat is required to decompose 1.35...
-
Use data from Table 7.3 and Appendix D to determine r H the following reaction. Table 7.3 Mg(OH)2(s) + 2NH4+ (aq) Mg2+ (aq) + 2 HO(1) + 2NH3(g) AH = ?
-
Twelve samples of a metal alloy are tested. The flexibility measurements had a sample average of 732.9 and a sample standard deviation of 12.5. (a) Is there sufficient evidence to conclude that the...
-
What is a rational engineering decision compared to an irrational decision? Explain
-
For 2020, Poly Scientific Inc. has reported earnings before interest and taxes (ebit) of $6.5 million; capital expenditures of $1.5 million; and depreciation of $0.7 million. Net working capital for...
-
Suppose that Jana cares only about apples and lettuce. Her utility function is U = A0.5L0.5, where A is the number of apples and L is the number of heads of lettuce that she consumes. The price of...
-
5. If the required reserve ratio is 10% and there is an initial deposit of $600, using the simple money multiplier, what is the maximum money created?
-
How much money will be in a saving account at the end of 10 years from deposits of $1500 per month. If the account earns interest at a rate of 12% per year compounded semiannually?
-
Zubac Company is a large retail furniture company that operates in two adjacent warehouses. One warehouse is a showroom, and the other is used to store merchandise. On the night of April 22, 2014, a...
-
In order to get an idea on current buying trends, a real estate agent collects data on 10 recent house sales in the area. Specifically, she notes the number of bedrooms in each house as follows: a....
-
Purchase of Computer with Zero-Interest-Bearing Debt Napoleon Corporation purchased a computer on December 31, 2009, for $130,000, paying $30,000 down and agreeing to pay the balance in five equal...
-
Asset Acquisition Logan Industries purchased the following assets and constructed a building as well. All this was done during the current year. Assets 1 and 2 These assets were purchased as a lump...
-
Nonmonetary Exchange Alatorre Corporation, which manufactures shoes, hired a recent college graduate to work in its accounting department. On the first day of work, the accountant was assigned to...
-
Let P R R be the orthogonal projection onto the hyperplane W = {(x, y, z, t) | 2t - (x + 2) = 0} (That is, the projection parallel to the normal vector (-1, 0, -1, 2).) Find the matrix M(P) of the...
-
Slippery Slope Roof Contracting has an equity beta of 1.2, capital structure with 2/3 debt, and a zero tax rate. What is their asset beta?
-
Assume that a bond will make payments every six months as shown on the following timeline: The timeline starts at Period 0 and ends at Period 18. It shows cash flows of $25.00 in each period from...

Study smarter with the SolutionInn App


