Question: Use data from Table 7.3 and Appendix D to determine r H the following reaction. Table 7.3 Mg(OH)2(s) + 2NH4+ (aq) Mg2+ (aq) +
Use data from Table 7.3 and Appendix D to determine ΔrH° the following reaction.
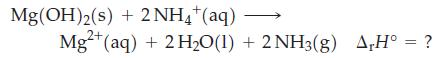
Table 7.3
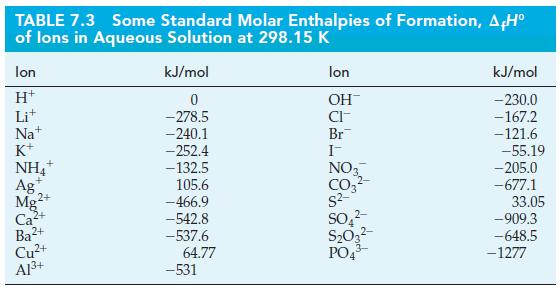
Mg(OH)2(s) + 2NH4+ (aq) Mg2+ (aq) + 2 HO(1) + 2NH3(g) AH = ?
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
MgOH2 2 NH 1 Mg 2HO 2 NH32 AH for MgOH2092454 KJmol AH for ... View full answer

Get step-by-step solutions from verified subject matter experts


