Question: Use data from Table 7.2, together with the fact that r H = -3509 kJ mol -1 for the complete combustion of pentane, C
Use data from Table 7.2, together with the fact that ΔrH° = -3509 kJ mol-1 for the complete combustion of pentane, C5H12(l), to calculate ΔrH° for the reaction below.
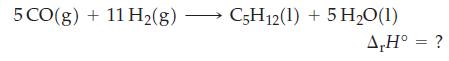
Table 7.2
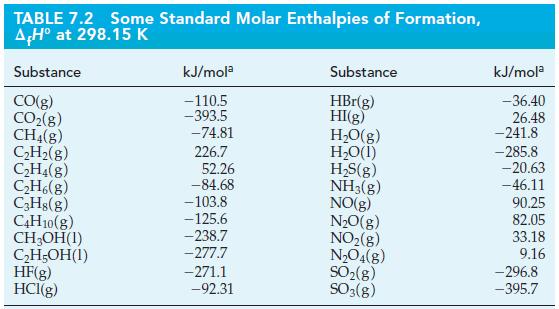
5 CO(g) + 11 H(g) - C5H12(1) + 5 HO(1) A,H = ?
Step by Step Solution
★★★★★
3.42 Rating (155 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

To determine the standard enthalpy change AH for the ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


