Question: Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL of liquid hydrazine at 25.0 C to hydrazine
Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL of liquid hydrazine at 25.0 °C to hydrazine vapor at its normal boiling point.
Integrative Example
Use data from the table of physical properties of hydrazine, N2H4, to calculate the partial pressure of N2H4(g) when a container filled with an equilibrium mixture of N2H4(g) and N2H4(I) at 25.0 °C is cooled to the temperature of an ice–water bath.
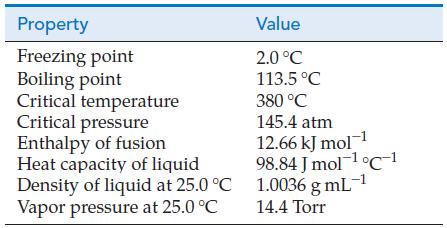
Property Freezing point Boiling point Critical temperature Critical pressure Enthalpy of fusion Heat capacity of liquid Density of liquid at 25.0 C Vapor pressure at 25.0 C Value 2.0 C 113.5 C 380 C 145.4 atm 12.66 kJ mol-1 98.84 Jmol- C 1 1.0036 g mL- 14.4 Torr
Step by Step Solution
There are 3 Steps involved in it
Heating the liquid hydrazine from 250 C to its normal boiling point of 1135 C Vaporizing the liquid hydrazine at its normal boiling point The heat req... View full answer

Get step-by-step solutions from verified subject matter experts


