Use the data in Appendix D to calculate the standard cell potential for each of the following
Question:
Use the data in Appendix D to calculate the standard cell potential for each of the following reactions. Which reactions will occur spontaneously?
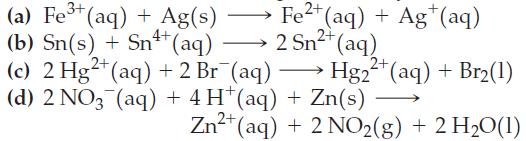
Transcribed Image Text:
2+ Fe2+ ²+ (aq) + Ag+ (aq) 2 Sn²+ (aq) Hg2 (a) Fe³+ (aq) + Ag(s) (b) Sn(s) + Sn++ (aq) (c) 2 Hg2+ (aq) + 2 Br¯(aq) →>> (d) 2 NO3(aq) + 4H+ (aq) + Zn(s) 2²+ (aq) + Br₂(1) - 2+ Zn²+ (aq) + 2 NO₂(g) + 2 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix D to calculate the standard cell potential for each of the following reactions. Which reactions will occur spontaneously? (a) H(g) + F2 (g) (b) Cu(s) + Ba+ (aq) 2 H(aq) + 2 F...
-
1. List out Linear and Non Linear data structures and explain them briefly. 2a) Write an algorithm for Bubble Sort.Also sort the folowing numbers using Bubble sort: 45, 23, 5, 41,5, 1, 20 2b) Sort...
-
Use the data in Appendix 3 to calculate the equilibrium constant for the reaction Agl(s) Ag+(aq) + I2(aq) at 25C. Compare your result with the Ksp value in Table 16.2.
-
3. How do the marginal costs of pollution reduction and the marginal costs of pollution damage change as pollution levels increase? The marginal costs of pollution reduction remain constant and the...
-
Discuss why Japanese distribution channels can be the epitome of blocked channels.
-
Why must the tax professional be cognizant of how tax law administration works?
-
Meal Cost A travel association claims that the mean daily meal cost for two adults traveling together on vacation in San Francisco is $105. A random sample of 20 such groups of adults has a mean...
-
Part 1: Firm A currently monopolizes its market and earns a profit of $10 million. Firm B is a potential entrant that is thinking about entering the market. If B does not enter the market, it earns a...
-
The trial balance of Skysong Wholesale Company contained the following accounts shown at December 31, the end of the companys fiscal year. SKYSONG WHOLESALE COMPANY Trial Balance December 31,2022...
-
Predict whether, to any significant extent, (a) Fe(s) will displace Zn 2+ (aq); (b) MnO 4 - (aq) will oxidize Cl - (aq) to Cl 2 (g) in acidic solution; (c) Ag(s) will react with 1 M HCl(aq); (d) O 2...
-
Predict whether the following metals will react with the acid indicated. If a reaction does occur, write the net ionic equation for the reaction. Assume that reactants and products are in their...
-
What were some of the causes of the stagflations of 1973 and 1979? In what ways were these episodes of stagflation different from the Great Depression of the 1930s?
-
Which of the five hazardous attitudes do you display most frequently? What can you do to minimize the presence and impact of these attitudes in your life?
-
What brought you to this course? How do you define Black or Blackness? What do you hope to get out of this class? When you think of Black Culture, what is the first thing that comes to mind? [For...
-
1. What is XBRL Taxonomy? How do you as a preparer of financial statement use the XBRL Taxonomy in locating a label for a specific financial element? 2. What are the benefits of adopting XBRL from...
-
What a business can do to protect and minimize the invasion of privacy for their customers? Think of your experience when visiting a website. What do most websites have you agree to before you do...
-
Based on your interest, skill set, or goals what do you typically contribute when working in groups? What do you need others to contribute due to your lack of interest, skill set, or goals? How do...
-
Access the FASB Accounting Standards Codification at the FASB website (asc.fasb.org). Determine the specific citation for accounting for each of the following items: 1. Initial measurement of stock...
-
You have accepted the engagement of auditing the financial statements of the C. Reis Company, a small manufacturing firm that has been your auditee for several years. Because you were busy writing...
-
Martinez, Inc. reported net income of $2.5 million in 2010. Depreciation for the year was $160,000, accounts receivable decreased $350,000, and accounts payable decreased $280,000. Compute net cash...
-
The net income for Adcock Co. for 2010 was $280,000. For 2010 depreciation on plant assets was $70,000, and the company incurred a loss on sale of plant assets of $12,000. Compute net cash provided...
-
The comparative balance sheets for Goltra Company show these changes in noncash current asset accounts: accounts receivable decrease $80,000, prepaid expenses increase $28,000, and inventories...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


