Question: Use the data in Appendix D to calculate the standard cell potential for each of the following reactions. Which reactions will occur spontaneously? (a) H(g)
Use the data in Appendix D to calculate the standard cell potential for each of the following reactions. Which reactions will occur spontaneously?
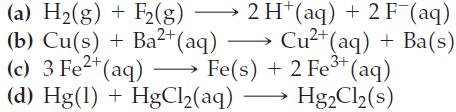
(a) H(g) + F2 (g) (b) Cu(s) + Ba+ (aq) 2 H(aq) + 2 F (aq) Cu+ (aq) + Ba(s) 3+ (c) 3 Fe+ (aq) Fe(s) + 2 Fe+ (aq) (d) Hg(1) + HgCl(aq) HgCl (s)
Step by Step Solution
★★★★★
3.47 Rating (157 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

To calculate the standard cell potential for each of the given reactions and determine which reactio... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


