Question: We saw that electronegativity differences determine whether bond dipoles exist in a molecule and that molecular shape determines whether bond dipoles cancel (nonpolar molecules) or
We saw that electronegativity differences determine whether bond dipoles exist in a molecule and that molecular shape determines whether bond dipoles cancel (nonpolar molecules) or combine to produce a resultant dipole moment (polar molecules). Thus, the ozone molecule, O3, has no bond dipoles because all the atoms are alike. Yet, O3 does have a resultant dipole moment: μ = 0.534 D. The electrostatic potential map for ozone is shown below. Use the electrostatic potential map to decide the direction of the dipole. Using the ideas of delocalized bonding in molecules, can you rationalize this electrostatic potential map?
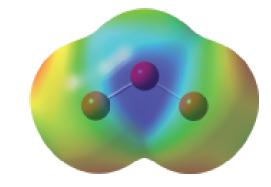
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
The electrostatic potential map of ozone shows that the two terminal oxygen atoms have a more negati... View full answer

Get step-by-step solutions from verified subject matter experts


