What formulas would you expect for the metal carbonyls of (a) Molybdenum, (b) Osmium; (c) Rhenium? Note
Question:
What formulas would you expect for the metal carbonyls of
(a) Molybdenum,
(b) Osmium;
(c) Rhenium?
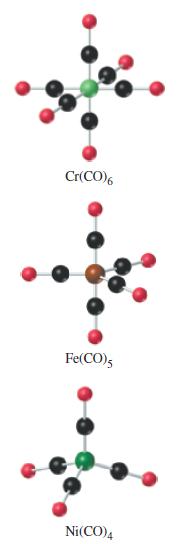
Note that the simple carbonyls shown in Figure 23-15 have one metal atom per molecule. Some metal carbonyls are binuclear; that is, they have two metal atoms bonded together in the carbonyl structure. Also,
(d) Explain why iron and nickel carbonyls are liquids at room temperature, whereas that of cobalt is a solid,
(e) Describe the probable nature of the bonding in the compound Na[V(CO)6].
Figure 23-15
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





