What is the (a) Degree of ionization and (b) Percent ionization of trichloroacetic acid in a 0.035
Question:
What is the
(a) Degree of ionization and
(b) Percent ionization of trichloroacetic acid in a 0.035 M CCl3COOH solution?
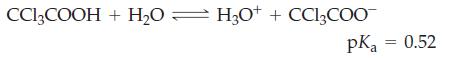
Transcribed Image Text:
CC13COOH + H₂0 ⇒ H3O+ + CCl₂COO pka 0.52 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To find the degree of ionization and percent ionization of trichloroacetic acid CCl3COOH in a 0035 M ...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Derive the form of the Langmuir isotherm when an adsorbing species occupies two surface sites, i.e: Diagrams included in the Solution
-
Exploring the degree of ionization equation in Study Question 127: Calculate the degree of ionization, , for formic acid at the following concentrations: 0.0100 M, 0.0200 M, 0.0400 M, 0.100 M, 0.200...
-
What is the (a) Degree of ionization and (b) Percent ionization of propionic acid in a solution that is 0.45 M CH 3 CH 2 CO 2 H? CH3CHCOH + HO H3O+ + CH3CHCO pka = 4.89
-
Refer to the adjusted trial balance for Romney's Marketing Company in M4-8. Prepare the closing entry at the end of the current year. M4-8 Romney's Marketing Company has the following adjusted trial...
-
How can the firm use currency options to hedge foreign-currency exposures resulting from international transactions?
-
The Berridge Company is a discount tire dealer that operates 25 retail stores in a metropolitan area. The company maintains a centralized purchasing and warehousing facility and employs a perpetual...
-
What is the purpose of practice aids in forensic and valuation services?
-
You are doing a review services and related tax work engagement for Murphy Construction Company. You have made extensive inquiries of management about their financial statements and have concluded...
-
The kibitzer computes the average of these three probabilities and announces the result of his computation publicly. Beatrice and Carol update their probabilities for F in the light of this new...
-
Continuing the dilutions described in Example 16-4, should we expect the percent ionization to be 13% in 0.0010 M CH 3 COOH and 42% in 0.00010 M CH 3 COOH? Explain. Example 16-4 What is the percent...
-
What must be the molarity of an acetic acid solution if it has the same percent ionization as 0.100 M CH 3 CH 2 CO 2 H (propionic acid, K a = 1.3 x 10 -5 )?
-
Why do short-term creditors, such as banks, emphasize balance sheet analysis when considering loan requests? Should they also analyze projected income statements? Why?
-
Blinder assumes that John and Jane take out a mortgage with a 20% down payment to invest in a $200,000 house that they expect to appreciate 20% over five years. In calculating their ROE over the...
-
ABC Company has two support departments (Power and Maintenance) and two producing departments (Assembly and Finishing). The direct allocation method is currently used to assign support department...
-
Lee Mealone is sledding with his friends. Disgruntled by a coarse comment, he decides to separate from the group. He momentarily exerts a 31 N force on the rope which is attached to his 2.5-kg sled....
-
Students will select a manufacturing or service firm listed on the New York Stock Exchange or NASDAQ approved by the instructor. Financial institutions and financial service firms are not to be used....
-
Your parents will retire in 2 7 years. They currently have $ 3 6 0 , 0 0 0 saved, and they think they will need $ 2 , 0 0 0 , 0 0 0 at retirement. What annual interest rate must they earn to reach...
-
Taylor Corp. is growing quickly. Dividends are expected to grow at a 30 percent rate for the next three years, with the growth rate falling off to a constant 6 percent thereafter. If the required...
-
What types of questions can be answered by analyzing financial statements?
-
A company reports the following: Net sales $560,000 Average accounts receivable (net) 40,000 Determine (a) The accounts receivable turnover and (b) The number of days sales in receivables. Round to...
-
A company reports the following: Net sales $600,000 Average accounts receivable (net) 60,000 Determine (a) The accounts receivable turnover and (b) The number of days sales in receivables. Round to...
-
A company reports the following: Cost of goods sold $510,000 Average inventory 60,000 Determine (a) The inventory turnover and (b) The number of days sales in inventory. Round to one decimal place
-
The following table shows values of a force function, f(x), where x is measured in meters and f(x) in newtons. Use simpsons Rute with 8 subintervals to estimate the work done by the force in moving...
-
Three point charges are located at the vertices of an equilateral triangle with sides 10 cm. The charges are q1= +2 mC, q2 = -4 mC, and q3 = -6 mC. Calculate the net force on each charge.. 10 cm 91...
-
Find the separation of two points on the Moon's surface that can just be resolved by the 5.10-m telescope on Palomar Mountain, assuming the separation is determined by diffraction effects and not...

Study smarter with the SolutionInn App


