What must be the molarity of an aqueous solution of trimethylamine, (CH 3 ) 3 N, if
Question:
What must be the molarity of an aqueous solution of trimethylamine, (CH3)3N, if it has a pH = 11.12?
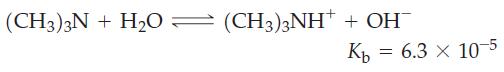
Transcribed Image Text:
(CH3)3 + H2O — (CH3)3NH' + OH Кь = 6.3 × 10-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
CH3 NHO CHNHOH Kb 63x10 and PH1112 According to HendersonHassalbalch ...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What must be the molarity of an aqueous solution of NH 3 if it is 4.2% ionized?
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
What must be the molarity of an acetic acid solution if it has the same percent ionization as 0.100 M CH 3 CH 2 CO 2 H (propionic acid, K a = 1.3 x 10 -5 )?
-
On March 20, Harbor's petty cash fund of $100 is replenished when the fund contains $19 in cash and receipts for postage $40, supplies $26, and travel expense $15. Prepare the journal entry to record...
-
Raymond Manufacturing faces a liquidity crisisit needs a loan of $100,000 for 1 month. Having no source of additional unsecured borrowing, the firm must find a secured short-term lender. The firms...
-
Waltman Co. juet ended its first year of operations. We are hired to help with the company's reporting. The Tableau Dashboard provides data for our analysin. Variable Manufacturing Costs Fixed...
-
Why does collusion pose unique prevention and detection challenges?
-
Mount Snow operates a Rocky Mountain ski resort. The company is planning its lift ticket pricing for the coming ski season. Investors would like to earn a 16% return on the companys $109,375,000 of...
-
As a successful entrepreneur and CEO, you are considering making some financial decisions for your engineering company. Examining the financial report of your company, you notice: . The upgrade of...
-
What are [H 3 O + ], [OH - ], pH, and pOH of 0.55 M M HClO 2 ?
-
What mass of benzoic acid, C 6 H 5 COOH, would you dissolve in 350.0 mL of water to produce a solution with a pH = 2.85? C6H5COOH + HO = H0+ + CHCOO Ka = 6.3 x 10-5
-
Selected cost data for Antique Print Co. are as follows: Estimated manufacturing overhead cost for the year .....$ 115,000 Estimated direct labor cost for the year .......... 71,875 Actual...
-
QUESTION 4: Evelyn Chiang & Gloria Fong Manufacturing (EGM) is considering replacing the existing manufacturing equipment with the new equipment. The existing equipment has a current market value of...
-
The real risk-free rate is 2%. Inflation is expected to be 3% this year, 4% next year, 5 in year 3 and then 3.5% thereafter. The liquidity premium for non-liquid assets amoutns to an average of...
-
Charles Henri is considering investing $37,800 in a project that is expected to provide him with cash inflows of $11,600 at the end of each of the first two years and $20,000 at the end of the third...
-
2) Calculate the GDP gap in Canada if the actual GDP is $600,000,000,000, the natural rate of unemployment is 5.4%, and the actual unemployment rate is 6.6%.
-
__________blank is considered a variable operating cost of an automobile. Multiple Choice Maintenance Interest on auto loan Insurance Registration fee DepreciationAna is trying to decide whether to...
-
(a) Find the amplitude, period, and phase shift of y = 3sin[(x 1/3)] (b) A ladder leans against a verfical wall of a building so that the angle between the ground and the ladder is 72 and its bottom...
-
Explain the term "Equivalent Units". Why are they calculated in process costing? [4 Marks] [minimum 350 words]
-
How are revenues and expenses reported on the income statement under (a) The cash basis of accounting and (b) The accrual basis of accounting?
-
Fees for services provided are billed to a customer during 2009. The customer remits the amount owed in 2010. During which year would the revenues be reported on the income statement under (a) The...
-
Employees performed services in 2009, but the wages were not paid until 2010. During which year would the wages expense be reported on the income statement under (a) The cash basis? (b) The accrual...
-
Alex was able to contribute to a 401K at his first job until the age of 30. The account had $24,000 when contributions stopped. The money continued to earn interest, compounded quarterly, at a rate...
-
4x3 = 0 In Exercises 15-22, solve the given linear system by any method. 15. 2x1 + x2 + 3x3 = 0 16. 2xy 3z = 0 - 3z= x1 + 2x2 = 0 -x+2y3z = 0 x2+x3 = 0 x+y+4z = 0
-
11. The table shows the results of rolling a die. Is the die fair? Explain. Number of Trials Experimental Probability of Rolling Each Number 1 2 3 4 5 6 4 9 40 or 0.10 or 0.23 40 40 404 or 0.10 40 40...

Study smarter with the SolutionInn App


