What mass of benzoic acid, C 6 H 5 COOH, would you dissolve in 350.0 mL of
Question:
What mass of benzoic acid, C6H5COOH, would you dissolve in 350.0 mL of water to produce a solution with a pH = 2.85?
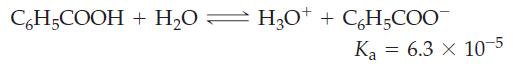
Transcribed Image Text:
C6H5COOH + H₂O = H₂0+ + C₂H₂COO™ Ka = 6.3 x 10-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To determine the mass of benzoic acid C6H5COOH required to produce a solution with a pH of 285 in 35...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
1. Sara borrowed funds to buy furniture for her business. Her business paying $200 at the end of each month for four years. The bank charges interest on the loan at 8% compounded monthly. a. What was...
-
The solubility of benzoic acid in water is: 0.29g/100mL at 20C 6.8g/100mL at 100C a) What is the maximum mass of benzoic acid that would crystallize out of solution if 8.0g of benzoic acid was...
-
(A) How many grams of (NH 4 ) 2 SO 4 must be dissolved in 0.500 L of 0.35 M NH 3 to produce a solution with pH = 9.00? (Assume that the solution volume remains at 0.500 L.) (B) In Practice Example...
-
Suppose the 2017 adidas financial statements contain the following selected data (in millions). Compute the following values and provide a brief interpretation of each. (a) Working capital. (b)...
-
Blair Finance factors the accounts of the Holder Company. All eight factored accounts are shown in the following table, with the amount factored, the date due, and the status on May 30. Indicate the...
-
Tom ONeal always wanted to own his own business. When he was in high school, he worked evenings and most weekends at a neighborhood bicycle shop. When Tom went to college at the nearby State...
-
What is the difference between a predator and a situational (accidental) fraudster?
-
1. Identify the ethical issues involved in the case from a consequentialist and deontological perspective (refer to Chapter 2). 2. Identify the management issues involved in the case. For example,...
-
Define the relational model. What is a relational database management system (DBMS)?
-
What must be the molarity of an aqueous solution of trimethylamine, (CH 3 ) 3 N, if it has a pH = 11.12? (CH3)3 + H2O (CH3)3NH' + OH = 6.3 10-5
-
Caproic acid, HC 6 H 11 O 2 , found in small amounts in coconut and palm oils, is used in making artificial flavors. A saturated aqueous solution of the acid contains 11 g/L and has pH = 2.94....
-
Define the difference between a "cash cost" and a "book cost." Is engineering economic analysis concerned with both types of cost? Give an example of each, and provide the context in which it is...
-
14)There are two independent dealers for Sporto automobiles in a large city. The dealers decide to run a cooperative advertising campaign in which both dealers are listed in local newspapers ads, and...
-
3. The TTC decided to increase its rates from $3.50 to $5.00. Currently ridership stands at 68,000 people a day. If Elasticity is 0.8, what would happen to total revenue? Would you advise the TTC to...
-
Angela and Betty are deciding how many nights to stay at a resort. Given above are the budget lines and indifference curves for both Angela and Betty. They are not travelling together and therefore...
-
A well-operated mass transit system in North America is one that obtains at least half its total revenues through the fare box (passenger fares). The remaining revenues are obtained as subsidies from...
-
The condensed statements of changes in financial position and detailed income statement information for Ivanhoe Consulting Ltd. follow. Ivanhoe contracts professionals in the electronic data...
-
Maryland Technical Acumen (MTA) is considering a new product line which will require an investment in production equipment and facilities in the current year. Below is an Income and cash flow...
-
What types of inventory issues Starbucks might reflect upon at the end of each year? The mission of Starbucks is to inspire and nurture the human spiritone person, one cup, and one neighborhood at a...
-
Is the matching concept related to (a) The cash basis of accounting or (b) The accrual basis of accounting?
-
Is the cash balance on the unadjusted trial balance the amount that should normally be reported on the balance sheet? Explain.
-
Is the supplies balance on the unadjusted trial balance the amount that should normally be reported on the balance sheet? Explain.
-
Use the following matrix. 0-1 2 A = 3 2 1 -5 3-1 What is the sum of matrix A and its negative? 1
-
Two people are painting a house. One person can paint the house by himself in 6 hours. The other person can paint the house by herself in 9 hours. How long will it take them to paint the house...
-
To own and operate a home printer, it costs $100 for the printer and an additional $0.05 per page for ink. To print out pages at an office store, it costs $0.25 per page. Let p represent number of...

Study smarter with the SolutionInn App


