What volume of H 2 (g) at 25 C and 752 mmHg is required to hydrogenate oleic
Question:
What volume of H2(g) at 25 °C and 752 mmHg is required to hydrogenate oleic acid, C17H33COOH(l), to produce one mole of stearic acid, C17H35COOH(s)? Assume reaction (22.52) proceeds with a 95% yield.
Reaction (22.52)
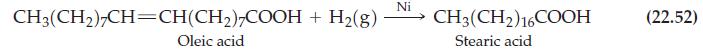
Transcribed Image Text:
CH3(CH₂)7CH=CH(CH₂)7COOH + H₂(g) Oleic acid Ni CH3(CH2) 16COOH Stearic acid (22.52)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To solve this problem we can use the following steps Write a balanced chemical equation for the reac...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) The same glass vessel used in Example 6-7 is filled with an unknown gas at 772 mmHg and 22.4 C. The gas-filled vessel weighs 40.4868 g. What is the molar mass of the gas? (B) A 1.27 g sample of...
-
Adelyn is in a financial dispute with her creditor. She wants to declare bankruptcy because she is finding herself unable to meet the requirements of paying off her debt. Which court that A would...
-
(A) The reaction of aluminum with hydrochloric acid produces hydrogen gas. The balanced chemical equation for the reaction is given below. 2 Al(s) + 6 HCl(aq) 2 AlCl 3 (aq) + 3 H 2 (g) If 35.5 mL of...
-
FIGURE P15.62 is a top view of an object of mass m connected between two stretched rubber bands of length L. The object rests on a frictionless surface. At equilibrium, the tension in each rubber...
-
(a) What is the purpose of internal earnings targets? (b) What is the risk associated with internal earnings targets?
-
Given these two exchange rates, $1 = 12.268 Mexican pesos and $1 = 0.7624, compute the cross rate between the Mexican peso and the euro. State this exchange rate in pesos and in euros.
-
Plaintiffs W. O. and J. C. Lucy had wanted to purchase Ferguson Farm from the Zehmers for at least eight years. One night, Lucy stopped by the establishment the Zehmers operated and said that he bet...
-
Santo Design Agency was founded by Thomas Grant in January 2004. Presented below is the adjusted trial balance as of December 31, 2008. Instructions (a) Prepare an income statement and a statement of...
-
You have been assigned to develop a data warehouse for a credit card company.The data source for the data warehouse will be a relational database consisting of four tables as specified below: Account...
-
CaH 2 (s) reacts with water to produce Ca(OH) 2 and H 2 (g). Ca(s) reacts with water to produce the same products. Na(s) reacts with water to form NaOH and H 2 (g). Without doing detailed...
-
Write equations to show how to prepare H 2 (g) from each of the following substances: (a) H 2 O; (b) HI(aq); (c) Mg(s); (d) CO(g). Use other common laboratory reactants as necessary, that is, water,...
-
Falco Services processes mortgage loan applications. The cost of home appraisals is included in its service fee, but Falco uses an outside appraisal service. The cost of appraisals has been...
-
A small sphere, with initial temperature \(T\), is immersed in an ideal Boltzmannian gas at temperature \(T_{0}\). Assuming that the molecules incident on the sphere are first absorbed and then...
-
Calculate the contribution of the first excited electronic state, namely \({ }^{1} \Delta\) with \(g_{e}=2\), of the \(\mathrm{O}_{2}\) molecule toward the Helmholtz free energy and the specific heat...
-
By considering the order of magnitude of the occupation numbers \(\left\langle n_{\varepsilon}ightangle\), show that it makes no difference to the final results of Section 7.1 if we combine a finite...
-
Last year, Miley decided to terminate the S corporation election of her solely owned corporation on October 17, 2018 (effective immediately), in preparation for taking it public. At the time of the...
-
In the data set on wolf upper jaws (Practice Problem 6), each measurement was actually the average of two measurements made on the left and right sides of the jaw of an individual wolf. Thus, a total...
-
Ivan and Olga own a duplex. They collect the rents and make repairs to the property when necessary. That is, they are active participants in the rental property. During the current year, the duplex...
-
Why is inventory management important for merchandising and manufacturing firms and what are the main tradeoffs for firms in managing their inventory?
-
Financial decisions often place heavier emphasis on one type of financial statement over the others. Consider each of the following hypothetical situations independently. (a) The North Face, Inc. is...
-
On June 1, Beardsley Service Co. was started with an initial investment in the company of $22,100 cash. Here are the assets and liabilities of the company at June 30, and the revenues and expenses...
-
Presented below is selected financial information for Yvonne Corporation for December 31, 2012. Instructions(a) Determine which items should be included in a statement of cash flows and then prepare...
-
The smallest mammal in the world. The smallest mammal in otherworld is the bumblebee bat, also known as a pig-nosed bat. These animals barely reach the size of a large bumblebee. The weights...
-
1. Use our definition of fractions and the math drawings below to give a detailed conceptual explanation for why of a ribbon is the same amount of ribbon as 23 2 X 4 3 X 4 of the ribbon. Discuss how...
-
Suppose the chance of rain in Chicago and Minneapolis are correlated. Define X = 1 if it is raining in Chicago some day and X = 0 if not; Similarly, define Y = 1 if it is raining in Minneapolis and Y...

Study smarter with the SolutionInn App


