When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm 3 )
Question:
When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm3) the barometric pressure is 748.2 mmHg, and the difference in mercury levels is 8.6 mmHg. What is the gas pressure Pgas?
Figure 6-5(c)
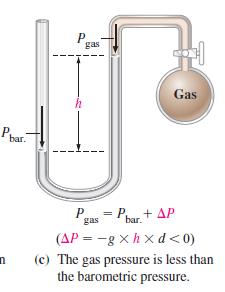
Transcribed Image Text:
Ph bar. n P h gas P gas 220 = Pbar + AP Gas (AP=-gxhxd<0) (c) The gas pressure is less than the barometric pressure.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Analyze We must first establish which is greater the barometric pressure or the ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Suppose that the mercury level in Example 6-2 is 7.8 mm higher in the arm open to the atmosphere than in the closed arm. What would be the value of P gas ? (B) Suppose P bar. and P gas are those...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A cylindrical glass beaker of height 1.520 m rests on a table. The bottom half of the beaker is filled with a gas, and the top half is filled with liquid mercury that is exposed to the atmosphere....
-
Briefly explain the differences between copyrights and patents.
-
Refer to the data and analysis developed for the Magee Company in Exhibits 8.5 and 8.6. Evaluate the alternatives using a cost of capital of 12 percent.
-
The following facts relate to McKane Corporation. 1. Deferred tax liability, January 1, 2015, \($60\),000. 2. Deferred tax asset, January 1, 2015, \($20\),000. 3. Taxable income for 2015,...
-
Rockstar Games, a subsidiary of Take-Two Interactive, released the video game Grand Theft Auto V in 2013. The game features a character named Lacey Jonas, a self-proclaimed actress slash singer and...
-
High Desert Potteryworks makes a variety of pottery products that it sells to retailers such as Home Depot. The company uses a job-order costing system in which predetermined overhead rates are used...
-
Supposons un consommateur ayant une richesse W qui est distribue selon une loi de densit de probabilit fw (w). Montrez qu'on peut obtenir une approximation du cot du risque, CR, en utilisant: 1 CRrr...
-
Calculate the height of a mercury column required to produce a pressure (a) Of 0.984 atm; (b) Of 928 Torr; (c) Equal to that of a column of water 142 ft high.
-
Explain how the action of a water siphon is related to that of a suction pump.
-
Factor each polynomial. x 3 + 64
-
The two basic forms of long-term liabilities are: Select one: a. Common shares and preferred shares b. Credit cards and operating lines of credit c. Loans and bonds d. Allowance for doubtful accounts...
-
Aiden has been operating a printing and stationery supply business since November 2010. In April 2023, he agreed to sell his business and the land and buildings on which it is located for a total...
-
Megan, age 40, has the following assets: $20,000 in mutual funds in an RRSP $17,500 in a non-registered 5-year GIC, purchased last year $38,400 in a LIRA $58,490 in an RPP Megan needs $12,000 to...
-
include all the correspondence auditors use with auditee personnel, officers, and third parties to conduct the audit; communication with management and those charged with governance includes the...
-
youll need to evaluate specific account values or financial statement paragraphs. As an analyst, you have access to the Securities and Exchange Commissions (SECs) EDGAR database of XBRL financial...
-
You are an assistant to a senator who chairs an ad hoc committee on reforming taxes on telecommunication services. Based on your research, AT& T has spent over $ 15 million on related paperwork and...
-
Willingness to pay as a measure of a person's value for a particular good measures the maximum a person would be willing to pay requires that payment actually be made depends on the satisfaction that...
-
Why didnt the FASB cover both types of postretirement benefitspensions and healthcarein the earlier pension accounting rules?
-
What are the major differences between postretirement healthcare benefits and pension benefits?
-
What is the difference between the APBO and the EPBO? What are the components of postretirement expense?
-
For any integer m and n, show that the polynomial x^3 + (5m+1)x + 5n +1 is irreducible over Z.
-
Suppose two marbles are drawn without replacement from a box with 1 blue, 3 white, 2 green, and 2 red. Find the probability that the second marble is red, given that the first marble is red.
-
A family is carpeting two rectangular rooms. They chose rugs that cost the same per square meter for each room. They estimated a cost of $400.00 to carpet their 12 foot by 15 foot bedroom. The living...

Study smarter with the SolutionInn App


