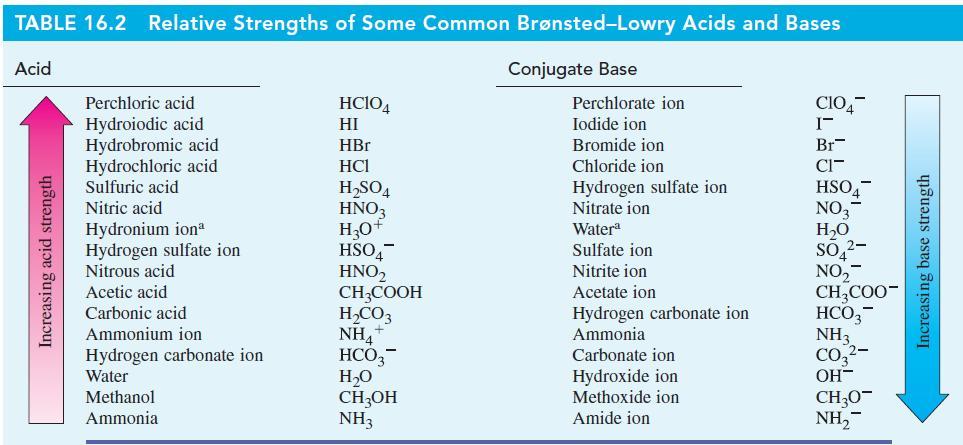
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the
Question:
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acid–base reactions.
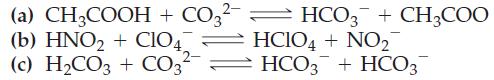
Table 16.2
Transcribed Image Text:
(a) CH3COOH + CO3²- = HCO3 + CH3COO (b) HNO₂ + ClO4 2- HCIO4 + NO₂ HCO3 + HCO3 (c) H₂CO3 + CO3²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To predict the direction favored in acidbase reactions you can consider the strengths of the acids a...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
60+ Reviews
338+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acidbase reactions. Table 16.2 (a) NH4+ + OH = HO + NH3 (b) HSO4 + NO3 HNO3 + SO4- (c) CH3OH +...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
With the aid of a periodic table (not Figure 9.15), arrange the following in order of increasing electronegativity: a. Sr, Ca, Rb b. Ca, Ge, Ga c. Se, As, Sb
-
Phoenix Corp. faltered in the recent recession but is recovering. Free cash flow has grown rapidly. Forecasts made in 2016 are as follows. Phoenix's recovery will be complete by 2021, and there will...
-
What is a revolving credit agreement? How does this arrangement differ from the line-of-credit agreement? What is a commitment fee?
-
A CVP graph, as illustrated on the shown below, is a useful technique for showing relationships between an organizations costs, volume, and profits. Required: 1. Identify the numbered components in...
-
Plaintiffs purchased stock warrants (rights to purchase) for blocks of Osborne Computer Corp., the manufacturer of the first mass-market portable personal computer. Because of inability to produce a...
-
Gregs Bicycle Shop has the following transactions related to its top-selling Mongoose mountain bike for the month of March 2015: Required: 1. Calculate ending inventory and cost of goods sold at...
-
Explain the FOUR ways you can map EERD to Relational Model?
-
(A) What is the pH of 0.015 M CH 2 FCOOH(aq)? (B) Piperidine is a base found in small amounts in black pepper. What is the pH of 315 mL of an aqueous solution containing 114 mg piperidine? CHFCOOH +...
-
Show by calculation that the pH of 0.100 M CH 3 COOH should be about the value shown on the pH meter in Figure 16-6; that is, pH 2.8. Figure 16-6 1.20 20 2.80. 19
-
The financial statements of Louis Vuitton are presented in Appendix F. Instructions for accessing and using the companys complete annual report, including the notes to its financial statements, are...
-
What information can you obtain from each of these statements? Examine Director's Resource 5-2: Income Statement, and Director's Resource 5-3: Statement of Financial Position.
-
A share of wells Fargo stock is paid a dividend of $3/share which is expected to grow at 2% annually. If the investor paid $1 for every share purchased at $40 and has 5% required rate of return, what...
-
IThe cost of replacing an existing building with a comparable substitute building, using modern construction methods and materials, is called:n Chapter eight we leIn the " Income Approach to Value"...
-
Hankins Corporation has 6.5 million shares of common stock outstanding, 322,000 shares of 4.7 percent preferred stock outstanding, par value of $100, and 75,000 5.5 percent semiannual bonds...
-
Wildly Successful Incorporated became so successful and profitable that on the advice of their accounting firm they decided to use a holding company when branching off certain services. Active...
-
Duchon Industries had the following balance sheet at the time it defaulted on its interest payments and filed for liquidation under Chapter 7. Sale of the fixed assets, which were pledged as...
-
1. Following are information about Alhadaf Co. Cost incurred Inventory Purchases Sales Adverting expense Salary Expense Depreciation Beginning Inventory Ending Inventory Amount 118,000 350.000 90,000...
-
Catherine Simpkins owns and operates Speedy Print Co. During February, Speedy Print Co. incurred the following costs in acquiring two printing presses. One printing press was new, and the other was...
-
Bridger Ski Co. has developed a tract of land into a ski resort. The company has cut the trees, cleared and graded the land and hills, and constructed ski lifts. (a) Should the tree cutting, land...
-
Fastball Delivery Company acquired an adjacent lot to construct a new warehouse, paying $30,000 and giving a short-term note for $270,000. Legal fees paid were $1,425, delinquent taxes assumed were...
-
with code examples and references for full credit. Consider an inheritance example apart from your textbook. Discuss your example and fully define all the classes, parent, and at least 3 derived...
-
If autozone's retained earnings and shareholder's equity was negative in 2 0 2 2 and 2 0 2 1 does that mean that autozone' has been experiencing poor financial performance? Operating lease...
-
Text: abatacababasababatasauhbana Pattern: abatasa Write down a simulation of finding one pattern in the text above using KMP string matching algorithm , i.e. write down the prefix table, the...

Study smarter with the SolutionInn App


