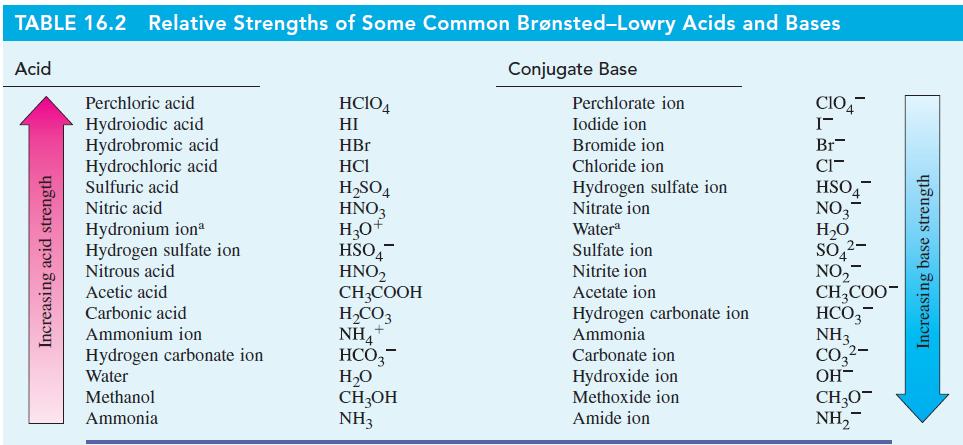
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the
Question:
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acid–base reactions.
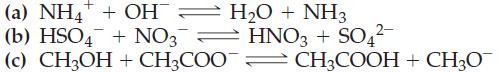
Table 16.2
Transcribed Image Text:
(a) NH4+ + OH = H₂O + NH3 (b) HSO4 + NO3 HNO3 + SO4²- (c) CH3OH + CH3COOCH3COOH + CH3O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acidbase reactions. Table 16.2 (a) CH3COOH + CO3- = HCO3 + CH3COO (b) HNO + ClO4 2- HCIO4 + NO...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
With the aid of a periodic table (not Figure 9.15), arrange the following in order of increasing electronegativity: a. Sr, Ca, Rb b. Ca, Ge, Ga c. Se, As, Sb
-
The CFO of the Jordan Microscope Corporation intentionally misclassified a downstream transportation expense in the amount of $575,000 as a product cost in an accounting period when the company made...
-
How is the prime rate of interest relevant to the cost of short-term bank borrowing? What is a floating-rate loan?
-
Kenworth Company uses a job-order costing system. Only three jobsJob 105, Job 106, and Job 107were worked on during November and December. Job 105 was completed on December 10; the other two jobs...
-
Brian Rafferty ingested finasteride, a drug prescribed to him to treat an enlarged prostate. Finasteride is a generic version of Proscar, a drug manufactured by Merck. Some time after he started to...
-
(Amortization SchedulesStraight-Line) Spencer Company sells 10% bonds having a maturity value of $3,000,000 for $2,783,724. The bonds are dated January 1, 2010, and mature January 1, 2015. Interest...
-
How might actions/events in other countries outside the U.S. effect the aggregate supply or aggregate demand in the U.S.? Be sure to explain.
-
The equation representing the neutralization of acetic acid, CH 3 COOH, by a base B is CH 3 COOH(aq) + B(aq) CH 3 COO - (aq) + BH + (aq). Of the bases listed in Table 16.4, which would be effective...
-
(A) Substituting halogen atoms for hydrogen atoms bound to carbon increases the strength of carboxylic acids. Show that the pH of 0.100 M CH 2 FCOOH, fluoroacetic acid, is lower than that calculated...
-
The table lists the annual amount (in billions of dollars) spent by the federal government on health research and training programs over a 10-yr period. Which one of the following provides the best...
-
A company borrows $50,000 by issuing a 6-month note payable with an annual interest rate of 8%. What is the total interest expense the company will incur over the term of the note?
-
Country Fixed Cost per Year Variable Cost per Population 1 $3,152,000 $65 2 $5,100,000 $35 3 $3,500,000 $55 4 $4,200,000 $45 Which location is best for serving a region with a population over 90,000?
-
The homemade dividend method might cost the shareholder money. What would need to happen to make this occur?
-
Q5. [4 points] Modify the method insert that you defined in the double linked list class DList. The modified method has the following definition. public static void insert2 (Node N, int pos) This...
-
A 1.80 m radius playground merry-go-round has a mass of 120.0 kg and is rotating freely (no friction) with an angular velocity of 0.290 rev/s. What is its angular velocity, in rev/s, after a 37.0 kg...
-
Write an essay in which you evaluate what a business or government agency would need to consider before transferring a hardy but non indigenous species to another country. Essay needs to be 3-4 pages...
-
What is an access control list?
-
A storage tank acquired at the beginning of the fiscal year at a cost of $172,000 has an estimated residual value of $20,000 and an estimated useful life of eight years. Determine the following: (a)...
-
Sandblasting equipment acquired at a cost of $85,000 has an estimated residual value of $5,000 and an estimated useful life of 10 years. It was placed in service on October 1 of the current fiscal...
-
A building with a cost of $1,050,000 has an estimated residual value of $420,000, has an estimated useful life of 36 years, and is depreciated by the straight-line method. (a) What is the amount of...
-
Single Taxable Income $0-$9,525 $9,526-$38,700 $38,701-$82,500 $82,501-$157,500 $157,501-$200,000 $200,001-$500,000 $500,001 or more Tax Rate 10% of taxable income $952.50 plus 12% of the amount over...
-
Prepare journal entries to record the following transactions. Saved a. Purchased $390 of supplies on credit. b. Completed $590 of work for a client on credit. c. Paid $390 cash towards the amount...
-
You need to purchase new tires for your car. The all-season light truck tires cost $90 each and are expected to last 30,000 miles. The premium brand on-off-road light truck tires cost $160 each....

Study smarter with the SolutionInn App


