Sodium azide, NaN 3 , undergoes the reaction NaN 3 (s) 2Na(s) + 3N 2 (g).
Question:
Sodium azide, NaN3, undergoes the reaction NaN3(s) → 2Na(s) + 3N2(g). Because this reaction is very fast and produces nitrogen gas, NaN3 is used to inflate airplane escape chutes. Sodium azide can be produced through two reaction steps.
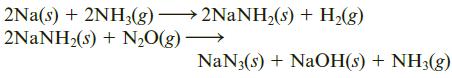
Starting with 1.0 kg of Na, 6.0 kg of NH3, and 1.0 kg of N2O, what is the maximum mass (kg) of sodium azide that can be produced?
Transcribed Image Text:
2Na(s) + 2NH3(g) 2NANH,(s) + H2(g) 2NANH2(s) + N,O(g) - NaN3(s) + NaOH(s) + NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
This problem is best approached by first combining the two steps given in the s...View the full answer

Answered By

Jehal Shah
I believe everyone should try to be strong at logic and have good reading habit. Because If you possess these two skills, no matter what difficult situation is, you will definitely find a perfect solution out of it. While logical ability gives you to understand complex problems and concepts quite easily, reading habit gives you an open mind and holistic approach to see much bigger picture.
So guys, I always try to explain any concept keeping these two points in my mind. So that you will never forget any more importantly get bored.
Last but not the least, I am finance enthusiast. Big fan of Warren buffet for long term focus investing approach. On the same side derivatives is the segment I possess expertise.
If you have any finacne related doubt, do reach me out.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each of the following indicate which reaction will occur faster. Explain your reasoning. (a) CH3CH2CH2CH2Br or CH3CH2CH2CH2I with sodium cyanide in dimethyl sulfoxide (b) 1-Chloro-2-methylbutane...
-
In each of the following indicate which reaction will occur faster. Explain your reasoning. (a) CH3CH2CH2CH2Br or CH3CH2CH2CH2I with sodium cyanide in dimethyl sulfoxide (b) 1-Chloro-2-methylbutane...
-
Starting with sodium azide as your source of nitrogen and using any other reagents of your choice, show how you would prepare each of the compounds in Problem 23.18. Compounds in 23.18 (a) (b) (c)...
-
What is the effect of a viscosity (competence) difference between strain markers and the matrix?
-
A bridge with a semielliptical arch spans a river as shown here. What is the clearance 6 ft from the riverbank? 14 ft 50 ft
-
What characteristics of Australia set its biodiversity apart from other global regions? Explain
-
Find an example of plot displaying geographic data and click on the image. You can answer the following questions using either the default variables and cases, or else use the menu on the left to...
-
Economist Lester Thurow once posed the following question: If you were the president of your own country and could choose one of two industries in which to specialize, computer chips or potato chips,...
-
In the beginning of January 2 0 2 3 you want to estimate the value of a stock. On December 3 1 2 0 2 2 the price of the stock was $ 6 3 . 5 and you believe that the annual dividend growth rate and...
-
XYZ is a calendar-year corporation that began business on January 1, 2020. For the year, it reported the following information in its current-year audited income statement. Notes with important tax...
-
Dinotrogen monoxide, commonly known as laughing gas, can be obtained by cautiously warming ammonium nitrate according to the equation NH 4 NO 3 (s) N 2 O(g) + 2H 2 O(g) If the reaction has a 75%...
-
A sample containing only boron and fluorine was decomposed yielding 4.75 mg of boron and 17.5 mL of fluorine gas (density = 1.43 g/L). What is the empirical formula of the sample compound?
-
(a) Use the vector dot product to show that in HCBr3, cos (BrCBr) = 1 - 1.5 sin2(HCBr). (b) In HCBr3, HCBr = 107.2. Find BrCBr in HCBr3.
-
The market price of a security is $92. Its expected rate of return is 20.1%. The risk-free rate is 3%, and the market risk premium is 6.4%. What will be the market price of the security if its...
-
3. Yvonne and Tanya each make regular deposits into an annuity. Yvonne deposits $150 at the end of each month at 8% per year compounded monthly Tanya deposits $450 at the end of each quarter at 8%...
-
The statement of assets and liabilities is - A . Balance sheet B . Trial balance C . Trading account D . Profit and loss account
-
You have been given the following projections for A&J Limited: _ 7 Year 1 2 3 4 5 ' Free cash flow of the firm 400 460 540 620 620 lntcrest-bearing debt 750 700 600 500 700 Interest expense 100 85 74...
-
The U.S. Central Intelligence Agency maintains an online World Factbook that is a convenient source of a wide variety of geographic, social, and economic information. You may look up information by...
-
a. Under the same conditions as those leading to the interval (7.5), P[(x = )/(/n) < 1.645] = .95. Use this to derive a one-sided interval for m that has infinite width and provides a lower...
-
What are the 5 Cs of marketing channel structure?
-
Hydrogen peroxide, H2O2, is a colorless liquid. A concentrated solution of it is used as a source of oxygen for rocket propellant fuels. Dilute aqueous solutions are used as a bleach. Analysis of a...
-
Nitric acid, HNO3, is a colorless, corrosive liquid used in the manufacture of nitrogen fertilizers and explosives. In an experiment to develop new explosives for mining operations, a sample...
-
Hydrogen cyanide, HCN, is a volatile, colorless liquid with the odor of certain fruit pits (such as peach and cherry pits). The compound is highly poisonous. How many molecules are there in 56 mg...
-
Multiplication of signed operands, which generate a double-length product in the 2's-complement 100 11 MULTIPLICAND (-13) 0 10 1 1 MULTIPLIER (+11) number system. The general strategy is the...
-
panel = Drawing Panel (160, 160) for i in range (0, 10): panel.draw rectangle (20, 20 + 100 10 * 1, 10 i, 10) Write variations of the above program that draw the figures at the lower right as output....
-
John estimated the following cash flows (in $) for a project: A B 1 Year Cash flow 2 0 -5,700 3 1 1,325 4 2 2,148 5 3 3,528 The required return for the project is 8%. What is the IRR for the project?

Study smarter with the SolutionInn App


