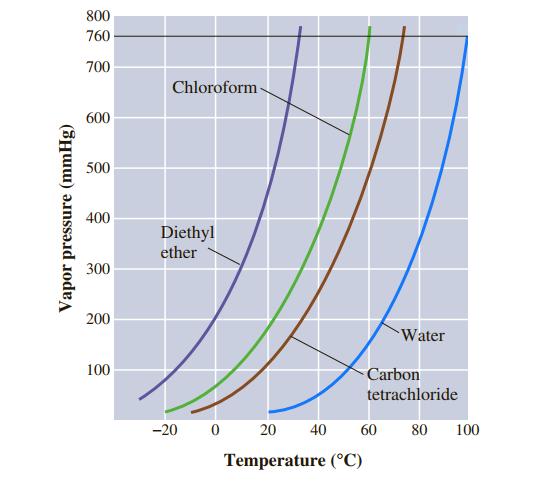
Use Figure 11.7 to estimate the boiling point of carbon tetrachloride, CCl 4, under an external pressure
Question:
Use Figure 11.7 to estimate the boiling point of carbon tetrachloride, CCl4, under an external pressure of 250 mmHg.
Transcribed Image Text:
800 760 700 Chloroform- 600 500 400 Diethyl ether 300 200 Water 100 Carbon tetrachloride -20 20 40 60 80 100 Temperature (°C) Vapor pressure (mmHg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
Dropping a line from the inter...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use Figure 11.7 to estimate the boiling point of diethyl ether, (C 2 H 5 ) 2 O, under an external pressure of 470 mmHg. 800 760 700 Chloroform- 600 500 400 Diethyl ether 300 200 Water 100 Carbon...
-
Using the vapor-pressure curves in Figure 11.25, (a) Estimate the boiling point of ethanol at an external pressure of 200 torr; (b) Estimate the external pressure at which ethanol will boil at 60 oC;...
-
Use Figure 10.8 to estimate the boiling point of ethanol at 400 torr.
-
a. Find the nth-order Taylor polynomials for the given function centered at the given point a, for n = 0, 1, and 2.b. Graph the Taylor polynomials and the function. f(x) = ln x, a e =
-
Suppose FRM, Inc., issued a zero coupon, equity index-linked note with a five-year maturity. The par value is $1,000, and the coupon payment is stated as 75 percent of the equity index return or as...
-
Write a program that asks the user to enter five test scores. The program should display a letter grade for each score and the average test score. Write the following methods in the program: ...
-
Consider the all-possible-regressions analysis of Hald's cement data in Example 10.1. If the objective is to develop a model to predict new observations, which equation would you recommend and why?...
-
The Trade Show Bureau conducted a survey to determine why people go to trade shows. The respondents were asked to rate a series of reasons on a scale from 1 to 5, with 1 representing little...
-
1. Describe the advantage of the creation of the Chong-Hua Institution for Economic Research. 2. What is the impact of tariffs imposed on Taiwan's imports by Mainland China? 3. Compare the Economic...
-
1. What are some of the primary reasons Netflix has been successful? 2. Netflix is a corporation. Why do you think the firm uses this form of ownership? 3. What threats might derail Netflix's...
-
Chloroform, CHCl 3 , a volatile liquid, was once used as an anesthetic but has been replaced by safer compounds. Chloroform boils at 61.7C and has a heat of vaporization of 31.4 kJ/mol. What is its...
-
An element crystallizes with a simple cubic lattice with atoms at all the lattice points. If the radius of the atom is 200. pm, what is the volume of the unit cell? a. 8.00 10 6 pm 3 b. 6.40 10 7...
-
Novak Corporation is preparing its 2012 statement of cash flows, using the indirect method. Presented below is a list of items that may affect the statement. Using the code below, indicate how each...
-
You are expected to do critique and conduct a literature review to discuss a contemporary issue which an IS professional may experience and identify appropriate approaches to address this issue. The...
-
Advise the directors of SAA on the concept of total cost of ownership TCO and how it can be successful applied? (25 marks)
-
You plan to invest $2,000 per year into a retirement account. If you earn a compound annual rate of return of 5%, how many years will it take you to reach a balance of $500,000? 50.14 47.22 51.99...
-
6 - 6 is The slope of the line y=5x- (Simplify your answer.)
-
Compute the distance between the points. (-10, -22) and (-18,-28) The distance between the given points is (Type an exact answer, using radicals as needed.)
-
Abby dies in the current year. In determining her Federal estate tax liability, comment on the relevance of each of the following. a. Abby made taxable gifts in 1975 and 2008. b. Abby held a life...
-
(a) What is the focal length of a magnifying glass that gives an angular magnification of 8.0 when the image is at infinity? (b) How far must the object be from the lens?
-
The bond length in C2 is 131 pm. Compare this with the bond lengths in C2H2 (120 pm), C2H4 (134 pm), and C2H6 (153 pm). What bond order would you predict for C2 from its bond length? Does this agree...
-
Draw resonance formulas of the nitric acid molecule, HNO3. What is the geometry about the N atom? What is the hybridization on N? Use bond energies and one Lewis formula for HNO3 to estimate Hf for...
-
One resonance formula of benzene, C6H6, is What is the other resonance formula? What is the geometry about a carbon atom? What hybridization would be used in valence bond theory to describe the...
-
= 1 and we i) Consider the function h(x) =x sin(x). We seek to find where h(x) will do so by using the bisection method to find the root of f(x) = h(x) 1. Use the intermediate value theorem to argue...
-
A major sports league, comprised of 200 players, reported a loss of $580 million this year. The sports league has yearly fixed costs of $700 million. The league is considering shutting down for the...
-
Use the one-period model to explain why a tax on capital is not a good idea. Determine the effects of capital tax on aggregate output, consumption, employment, and the real wage. Please use diagrams...

Study smarter with the SolutionInn App


