a) Show that the problem of flash vaporization of a multicomponent ideal mixture can be reformulated as
Question:
a) Show that the problem of flash vaporization of a multicomponent ideal mixture can be reformulated as suggested by Rachford and Rice (Doherty and Malone, 2001):
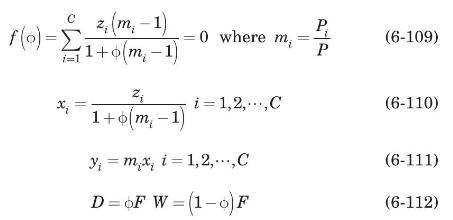
Equation (6-109) is solved iteratively for \(\phi\); all other variables are calculated explicitly from equations (6-110) to (6-112).
(b) Solve Example 6.2 using the Rachford-Rice method.
Data From Example 6.2:-
A liquid containing 50 mol% benzene (A), 25 mol% toluene (B), and 25 mol% o-xylene (C) is flash-vaporized at 1 atm and 373 K. Compute the amounts of liquid and vapor products and their composition. These components form ideal mixtures. The vapor pressures of the three components at 373 K are PA = 178.8 kPa, PB = 73.6 kPa, and PC = 26.3 kPa. Therefore, for a total pressure of 1 atm and a temperature of 373 K, mA = 1.765, mB = 0.727, mC = 0.259.
Step by Step Answer:






