In the treatment of wastewater, undesirable gases are frequently stripped or desorbed from the water, and oxygen
Question:
In the treatment of wastewater, undesirable gases are frequently stripped or desorbed from the water, and oxygen is adsorbed into the water when bubbles of air are dispersed near the bottom of aeration tanks or ponds. As the bubbles rise, solute can be transferred from the gas to the liquid or from the liquid to the gas, depending upon the concentration driving force.
For batch aeration in a constant-volume tank, an oxygen mass balance can be written as
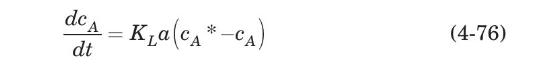
where $c_{A}{ }^{*}$ is the oxygen saturation concentration. Integrating between the time limits zero and $t$ and the corresponding dissolved oxygen concentration limits $c_{A, 0}$ and $c_{A, t}$, and assuming that $c_{A} *$ remains essentially constant and that all the resistance to mass transfer resides in the liquid phase, we obtain
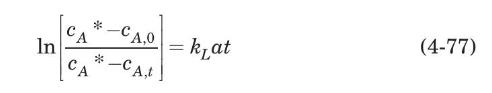
In aeration tanks, where air is released at an increased liquid depth, the solubility of oxygen is influenced both by the increasing pressure of the air entering the aeration tank and by the decreasing oxygen partial pressure in the air bubble as oxygen is absorbed. For these cases, the use of a mean saturation value corresponding to the aeration tank mid-depth is suggested (Eckenfelder, 2000):
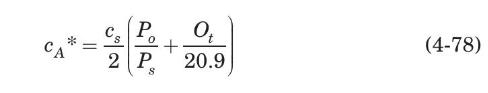
where

The molar oxygen percent in the air leaving the aeration tank is related to the oxygen transfer efficiency, $O_{\text {eff }}$, through
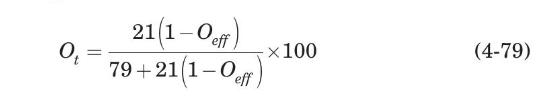
where
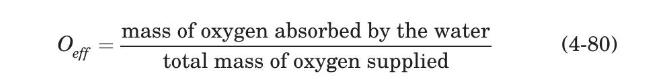
Consider a $567-\mathrm{m}^{3}$ aeration pond aerated with 15 spargers, each using compressed air at a rate of $0.01 \mathrm{~kg} / \mathrm{s}$. Each sparger is in the form of a ring $100 \mathrm{~cm}$ in diameter, containing 20 orifices, each $3.0 \mathrm{~mm}$ in diameter. The spargers will be located $5 \mathrm{~m}$ below the surface of the pond. The water temperature is $298 \mathrm{~K}$; atmospheric conditions are $298 \mathrm{~K}$ and $101.3 \mathrm{kPa}$. Under these conditions, $c_{s}=8.38 \mathrm{mg} / \mathrm{L}$ (Davis and Cornwell, 1998).
(a) Estimate the volumetric mass-transfer coefficient for these conditions from equations (4-28) and (4-30).
(b) Estimate the time required to raise the dissolved oxygen concentration from $0.5 \mathrm{mg} / \mathrm{L}$ to $6.0 \mathrm{mg} / \mathrm{L}$ and calculate the resulting oxygen transfer efficiency.
(c) Estimate the power required to operate the 15 spargers if the mechanical efficiency of the compressor is $60 \%$.
Data From Equations 4-28 and 4-30:-

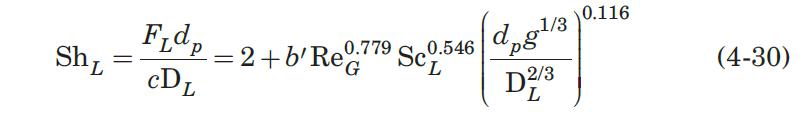
Step by Step Answer:






