Nitric oxide (NO) emissions from automobile exhaust can be reduced by using a catalytic converter, and the
Question:
Nitric oxide (NO) emissions from automobile exhaust can be reduced by using a catalytic converter, and the following reaction occurs at the catalytic surface:
\[\mathrm{NO}+\mathrm{CO} \rightarrow \frac{1}{2} \mathrm{~N}_{2}+\mathrm{CO}_{2}\]
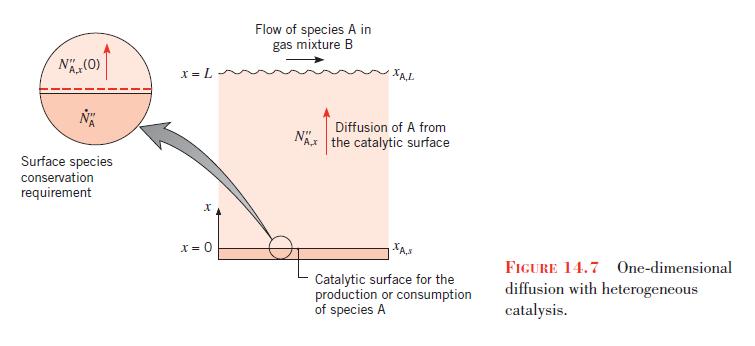
The concentration of NO is reduced by passing the exhaust gases over the surface, and the rate of reduction at the catalyst is governed by a first-order reaction of the form given by Equation 14.66. As a first approximation it may be assumed that NO reaches the surface by one-dimensional diffusion through a thin gas film of thickness \(L\) that adjoins the surface. Referring to Figure 14.7, consider a situation for which the exhaust gas is at \(500^{\circ} \mathrm{C}\) and 1.2 bar and the mole fraction of \(\mathrm{NO}\) is \(x_{\mathrm{A}, L}=0.10\). If \(D_{\mathrm{AB}}=10^{-4} \mathrm{~m}^{2} / \mathrm{s}, k_{1}^{\prime \prime}=0.05 \mathrm{~m} / \mathrm{s}\), and the film thickness is \(L=1 \mathrm{~mm}\), what is the mole fraction of \(\mathrm{NO}\) at the catalytic surface and what is the NO removal rate for a surface of area \(A=250 \mathrm{~cm}^{2}\) ?
Data From Figure 14.7:-
Equation 14.66:-

Step by Step Answer:

Fundamentals Of Heat And Mass Transfer
ISBN: 9781119220442
8th Edition
Authors: Theodore L. Bergman, Adrienne S. Lavine





