The latent heat of vaporization per unit mass of a pure substance at a given temperature, (lambda),
Question:
The latent heat of vaporization per unit mass of a pure substance at a given temperature, \(\lambda\), is defined as the difference in enthalpy between the saturated vapor and saturated liquid at the given temperature, \(T\). Since enthalpy is a thermodynamic function of state, show that \(\lambda\) can be evaluated from a known value of \(\lambda_{0}\) at a reference temperature \(T_{0}\) from the equation
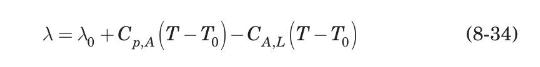
where \(C_{p, A}\) and \(C_{A, L}\) are, respectively, the average heat capacities of gas and liquid between \(T_{0}\) and \(T\).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





