(a) Na 3 N remained an elusive compound until 2002. Calculate a value for f H...
Question:
(a) Na3N remained an elusive compound until 2002. Calculate a value for ΔfHo(Na3N, s) using data from Appendices 8 and 10, and the following estimated values of ΔH(298 K):
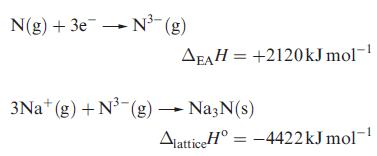
Comment on whether the value obtained is sufficient to indicate whether Na3N is thermodynamically stable.
(b) The high-temperature crystalline form of RbNH2 adopts a structure with a ccp array of [NH2]‾ ions and Rb+ ions occupying octahedral sites. To which structure type does this correspond? Sketch a unit cell of RbNH2 and confirm the stoichiometry of the compound by considering the number of ions per unit cell.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





