Question: (a) Using standard reduction potentials from Appendix 11, determine values of f G(K + , aq) and f G(F, aq). (b) Hence, find
(a) Using standard reduction potentials from Appendix 11, determine values of ΔfGº(K+, aq) and ΔfGº(F‾, aq).
(b) Hence, find ΔsolGº(KF, s) at 298 K, if ΔfGº(KF, s) = –537.8 kJ mol–1.
(c) What does the value for ΔsolGº(KF, s) imply about the solubility of KF in water?
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of Eo refers to [OH−] = 1 mol dm−3, hence the notation Eo[OH−] = 1
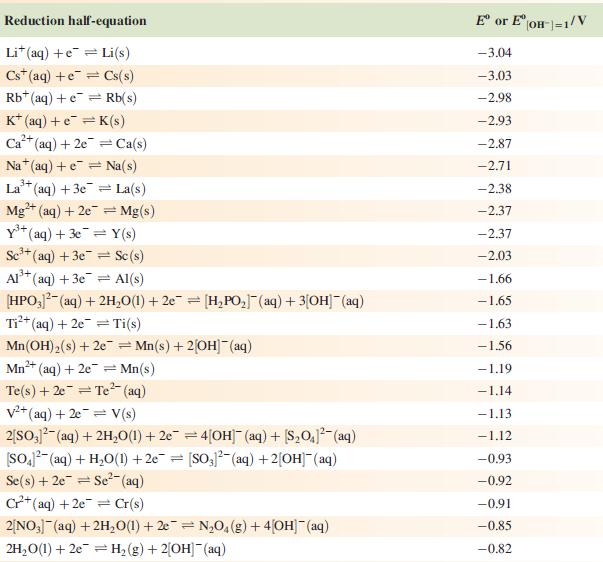

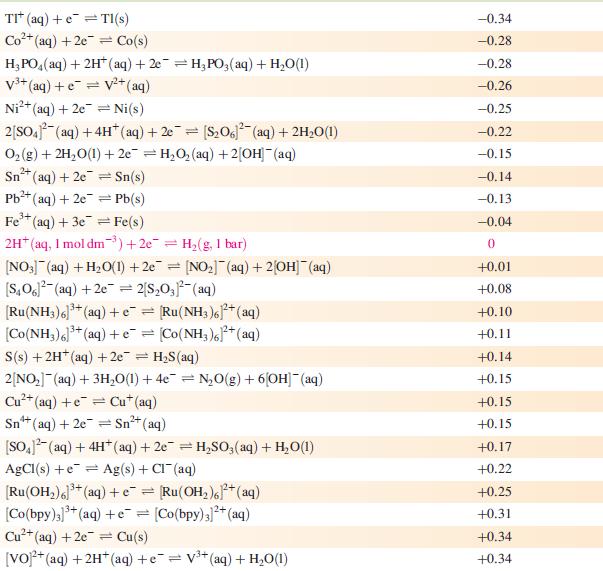
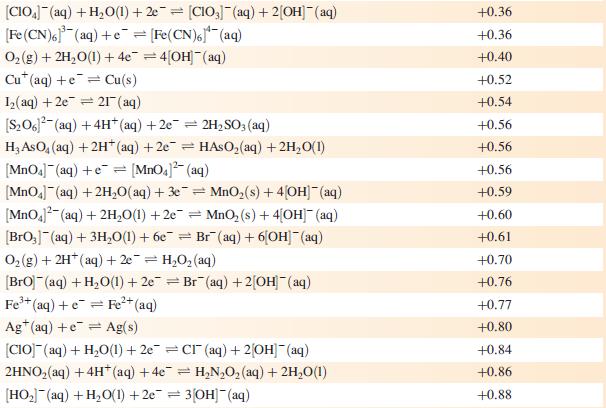
![Al(s) Al+ (aq) + 3e [HPO3)(aq) + 2HO(1) + 2e [HPO] (aq)](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2023/07/64bcdb1d03faa_46064bcdb1c6cb23.jpg)

Reduction half-equation Li (aq) +eLi(s) Cst (aq) +eCs(s) Rb+ (aq) + e Rb(s) K+ (aq) + eK(s) 2+ Ca+ (aq) + 2e Ca(s) Na (aq) + e Na(s) 3+ La+ (aq) + 3e = 2+ Mg+ (aq) + 2e Y+ (aq) + 3eY(s) Se+ (aq) + 3e Sc(s) Al(s) Al+ (aq) + 3e [HPO3)(aq) + 2HO(1) + 2e [HPO] (aq) + 3[OH(aq) = = La(s) = Mg(s) Ti+ (aq) + 2e Ti(s) Mn(OH) (s) +2e=Mn(s) + 2OH(aq) Mn+ (aq) + 2e = Mn(s) 2+ Te(s) + 2e Te (aq) = = V+ (aq) + 2e = V(s) 2[SO3)(aq) + 2HO(1) +2e=4[OH] (aq) + [S01(aq) [SO4- (aq) + HO(1) +2e= [SO3)2(aq) + 2[OH] (aq) Se(s) + 2e Se- (aq) C+ (aq) +2e Cr(s) 2[NO3] (aq) + 2HO(1) +2e=NO4(g) + 4[OH](aq) 2HO(1) + 2e = H(g) + 2[OH]- (aq) E or E" (OH-]=1/V -3.04 -3.03 -2.98 -2.93 -2.87 -2.71 -2.38 -2.37 -2.37 -2.03 -1.66 -1.65 -1.63 -1.56 -1.19 -1.14 -1.13 -1.12 -0.93 -0.92 -0.91 -0,85 -0.82
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
To find the standard free energy of formation for Kaq and Faq we will use their standard reduction potentials along with the standard reduction potent... View full answer

Get step-by-step solutions from verified subject matter experts


