In their article Enzymes and bio-inspired electrocatalysts in solar fuel devices (Energy Environ. Sci., 2012, 5, 7470),
Question:
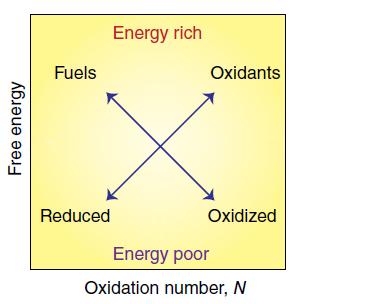
In their article ‘Enzymes and bio-inspired electrocatalysts in solar fuel devices’ (Energy Environ. Sci., 2012, 5, 7470), Woolerton et al. use a generic Frost diagram (Fig. 6.21) to illustrate the concept of chemical energy storage and release. So-called ‘energy-rich substances’ are compounds (fuels and oxidants) in which chemical energy is stored: fuels (such as hydrocarbons, boranes, metal hydrides) lie towards the upper left whereas strong oxidants such as O2 lie towards the upper right. ‘Energy-poor substances’ are stable, reduced compounds (e.g. H2O) lying towards the lower left and oxidized compounds (e.g. CO2, components of ash) lying towards the lower right. Energy release (whether by combustion or in a fuel cell or battery) occurs when a species from the upper left reacts with a species from the upper right to give products positioned diagonally down their respective arrows. During energy storage (such as photosynthesis or charging a battery) compounds from the lower left and lower right are transformed using sunlight or electricity into products that are positioned diagonally upwards along their respective arrows. Using data from Resource section 3 assess the usefulness of this concept for a methanol/oxygen system and a lead–acid battery.
Figure 6.21.
Step by Step Answer:

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke





