In their article Some observations on molecular orbital theory (J.F. Harrison and D. Lawson, J. Chem. Educ.,
Question:
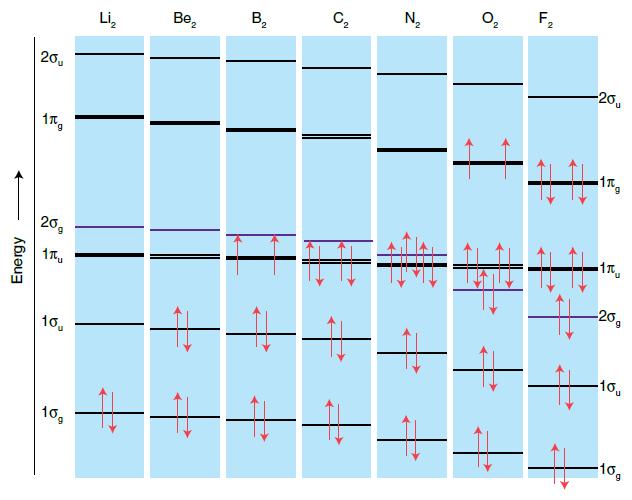
In their article ‘Some observations on molecular orbital theory’ (J.F. Harrison and D. Lawson, J. Chem. Educ., 2005, 82, 1205) the authors discuss several limitations of the theory. What are these limitations? Sketch the MO diagram for Li2 given in the paper. Why do you think this version does not appear in textbooks? Use the data given in the paper to construct MO diagrams for B2 and C2. Do these versions differ from those in Fig. 2.17 in this textbook? Discuss any variations.
Figure 2.17.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





